2010 New Drug Approvals - part VIII - Cabazitaxel (Jevtana)
ATC code (partial): L01CD
The FDA approved Cabazitaxel on June 17th. Cabitaxel is used in combination with prednisone to treat advanced, hormone-refractory prostate cancer.
Typically, prostate cancer occurs in older males and is among the most common forms of cancer affecting men with around 200,000 new cases per year diagnosed in the United States. Prostate cancer is a glandular cancer that is generally slow-growing. However, prostate cancer develops the ability to penetrate into other tissues and to form metastases.
Cabazitaxel is a new antineoplastic agent that inhibits the function of microtubules. Like other taxanes, it binds to beta-tubulin and promotes and maintains its incorporation in the assembled microtubule. As a consequence the dynamic structure of the microtubule cytoskeleton is 'frozen' and the concentration of free tubulin decreased. Mitotic cells, which depend on microtubules to restructure their shape and organelle organization, undergo apoptosis or stop progressing through the cell cycle. Thus, tumor growth is stalled.
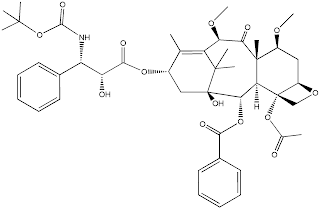
Chemically, cabazitaxel is a dimethylated variant of the state-of-the-art treatment docetaxel. Cabazitaxel is an example of a semin-synthetic natural product, with the key raw ingredient isolated from yew leaves. Cabazitaxel is a lipophillic molecule with a high molecular weight 835.93 g.mol-1).
The volume of distribution (Vss) is 4,864 L. In vitro, the binding of cabazitaxel to human serum proteins was 89 to 92%. Cabazitaxel is mainly bound to human serum albumin (82%) and lipoproteins (88% for HDL, 70% for LDL, and 56% for VLDL). Cabazitaxel is extensively metabolized in the liver (> 95%), mainly by the CYP3A4/5 isoenzyme, and to a lower extent by CYP2C8. Clearance (primarily as metabolites) is via both urine and feces. Cabazitaxel has a plasma clearance of 48.5 L.hr-1.
Clinical trials with showed a statistically significant increase in survival rate by 2.4 month.
Cabazitaxel is to be administered as an intravenous infusion of 25mg/mm2(body surface) every three weeks in combination with a daily regiment of 10mg prednisone.
Cabazitaxel comes with a black box warning (fatal neutropenia). Patients have to be monitored for changes in neutrophil counts and treatment appropriately adjusted if a drop below 1500 neutrophils/mm3 is observed. Other adverse side effects of cabazitaxel are hypersensivity reactions, gastrointestinal symptoms and renal failure.
Cabazitaxel is marketed by Sanofi-Aventis under the name Jevtana. This is a link to the full prescribing information.
SMILES:
CO[C@H]1C[C@H]2OC[C@@]2(OC(C)=O)[C@H]3[C@H](OC(=O)c4ccccc4)[C@]5(O)C
[C@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)c6ccccc6)C(=C([C@@H](OC)C
(=O)[C@]13C)C5(C)C)C
InChI:
1S/C45H57NO14/c1-24-28(57-39(51)33(48)32(26-17-13-11-14-18-26)46-40
(52)60-41(3,4)5)22-45(53)37(58-38(50)27-19-15-12-16-20-27)35-43(8,
36(49)34(55-10)31(24)42(45,6)7)29(54-9)21-30-44(35,23-56-30)59-25(2)
47/h11-20,28-30,32-35,37,48,53H,21-23H2,1-10H3,(H,46,52)/t28-,29-,30+,
32-,33+,34+,35-,37-,43+,44-,45+/m0/s1
InChI-Key:
BMQGVNUXMIRLCK-OAGWZNDDSA-N
Chemdraw: cabazitaxel.cdx