New Drug Approvals 2011 - Pt. IV Human coagulation Factor XIII Concentrate (CorifactTM)
Prior to US approval, FXIII Concentrate had already been marketed in Europe, as Fibrogammin P, developed by CSL Behring of Marburg, Germany. Recently, recombinant FXIII (rFXIII, Novo Nordisk, Bagsværd, Denmark) has completed phase 3 clinical trials.
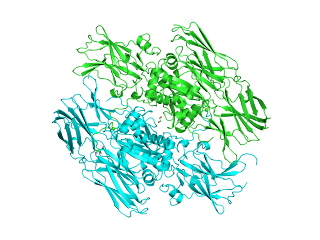
FXIII exists naturally as a secreted, tetrameric protein (with composition A2B2) with a molecular weight of ca. 320 kDa. The tetramer consists of twice two catalytically active transglutaminase (A subunit, Uniprot:P00488) (E.C. 2.3.2.13) and an enzymatically inactive carrier (B subunit, Uniprot:P05160) subunits in the blood plasma, or as a catalytically active dimer (A2) inside platelets, monocytes, macrophages and their respective precursor cells. The B-chain holds the A chain dimer in an inactive state, and also shields the A-chain from proteolytic degradation in the plasma.
>Corifact A chain GVNLQEFLNVTSVHLFKERWDTNKVDHHTDKYENNKLIVRRGQSFYVQIDFSRPYDPRRD LFRVEYVIGRYPQENKGTYIPVPIVSELQSGKWGAKIVMREDRSVRLSIQSSPKCIVGKF RMYVAVWTPYGVLRTSRNPETDTYILFNPWCEDDAVYLDNEKEREEYVLNDIGVIFYGEV NDIKTRSWSYGQFEDGILDTCLYVMDRAQMDLSGRGNPIKVSRVGSAMVNAKDDEGVLVG SWDNIYAYGVPPSAWTGSVDILLEYRSSENPVRYGQCWVFAGVFNTFLRCLGIPARIVTN YFSAHDNDANLQMDIFLEEDGNVNSKLTKDSVWNYHCWNEAWMTRPDLPVGFGGWQAVDS TPQENSDGMYRCGPASVQAIKHGHVCFQFDAPFVFAEVNSDLIYITAKKDGTHVVENVDA THIGKLIVTKQIGGDGMMDITDTYKFQEGQEEERLALETALMYGAKKPLNTEGVMKSRSN VDMDFEVENAVLGKDFKLSITFRNNSHNRYTITAYLSANITFYTGVPKAEFKKETFDVTL EPLSFKKEAVLIQAGEYMGQLLEQASLHFFVTARINETRDVLAKQKSTVLTIPEIIIKVR GTQVVGSDMTVTVQFTNPLKETLRNVWVHLDGPGVTRPMKKMFREIRPNSTVQWEEVCRP WVSGHRKLIASMSSDSLRHVYGELDVQIQRRPSM
>Corifact B chain EEKPCGFPHVENGRIAQYYYTFKSFYFPMSIDKKLSFFCLAGYTTESGRQEEQTTCTTEG WSPEPRCFKKCTKPDLSNGYISDVKLLYKIQENMRYGCASGYKTTGGKDEEVVQCLSDGW SSQPTCRKEHETCLAPELYNGNYSTTQKTFKVKDKVQYECATGYYTAGGKKTEEVECLTY GWSLTPKCTKLKCSSLRLIENGYFHPVKQTYEEGDVVQFFCHENYYLSGSDLIQCYNFGW YPESPVCEGRRNRCPPPPLPINSKIQTHSTTYRHGEIVHIECELNFEIHGSAEIRCEDGK WTEPPKCIEGQEKVACEEPPFIENGAANLHSKIYYNGDKVTYACKSGYLLHGSNEITCNR GKWTLPPECVENNENCKHPPVVMNGAVADGILASYATGSSVEYRCNEYYLLRGSKISRCE QGKWSSPPVCLEPCTVNVDYMNRNNIEMKWKYEGKVLHGDLIDFVCKQGYDLSPLTPLSE LSVQCNRGEVKYPLCTRKESKGMCTSPPLIKHGVIISSTVDTYENGSSVEYRCFDHHFLE GSREAYCLDGMWTTPPLCLEPCTLSFTEMEKNNLLLKWDFDNRPHILHGEYIEFICRGDT YPAELYITGSILRMQCDRGQLKYPRCIPRQSTLSYQEPLRT
Several crystal structures are available for the catalytically active A chain dimer of FXIII, e.g. PDBe:1EVU.
FXIII is activated by proteolytic cleavage of the first 37 N-terminal amino acids by thrombin (also known as factor II). In presence of calcium ions, the carrier subunits dissociate, leading to a conformational change exposing the catalytic center of the A chain, thus capable of crosslinking of fibrin molecules to form an insoluble clot. In order to restore natural coagulaton, patients suffering from FXIII deficiency can therefore be treated with exogenous FXIII.
The half-life (t1/2) of Corifact is 6.6 days, with a steady state Volume of distribution (Vss) of 52 mL.kg-1, and a Clearance (Cl) of 0.25 mL.hr-1.kg-1.
Corifact is made from pooled human donor blood plasma, and is supplied as lyophilized concentrate for intravenous administration after reconstitution with sterile water. The recommended initial dosing is specific to the patients body weight and existing blood coagaultion parameters. Typical dosage is 40 IU per kg body weight and is repeated every 28 days. In the subsequent dosing, FXIII activity levels are monitored, and adjusted to achieve the intended trough FXIII activity level. As a human blood product, the production process of Corifact is monitored to minimize possible contamination with virus (e.g., HIV, HAV, HBV, and HCV), and the infective agent of CJD.
The full prescribing information can be found here.
The license holder for Corifact is CSL Behring, and the product website is here.