New Drug Approvals 2011 - Pt. VI Roflumilast (DalirespTM)
On February 28th, 2011, the FDA approved Roflumilast (tradename:Daxas tradename:Daliresp NDA 022522) for the treatment of patients with chronic obstructive pulmonary disease (COPD) a chronic and serious disease involving restriction of full lung function. The narrowing of airways of COPD is irreversible, and follows inflammation in the lung, believed to be linked to environmental pollutants such as tobacco smoke, workplace dusts and urban air pollution. This inflammation causes structural damage to the delicate alveoli structures.
Roflumilast and an active metabolite, Roflumilast-N-Oxide, are selective Phosphodiesterase 4 inhibitors. The subfamily of Type 4 Phospodiesterases comprises four distinct members, PDE4A, -4B, -4C, and -4D (Uniprot:P27815, Q07343, Q08493, Q08499, respectively, all are very closely related enzymes containing a characteristic cyclic nucleotide diesterase catalytic domain Pfam:PF00233). These in turn occur in different splicing isoforms with tissue specific expression, many of them in the lung. Phosphodiesterase 4 catalyzes a reaction transforming cyclic 3'-5'-adenosine monophosphate (cAMP, ChEBI: 17489) into adenosine 5'-monophosphate (AMP). Roflumilast has an IC50 against PDE-4 of ca. 2nM, affinities against the PDE4A, PDE4B, and PDE4D isozymes are all similar, whereas affinity against the PDE4C isozyme is ca. 5 fold lower. The exact mechanism by which Roflumilast reduces the risk of COPD exacerbations is not known, but it is believed that an increase in cAMP levels in lung cells attenuates the abnormal inflammation process associated with COPD. In clinical trials, it was observed that the numbers of specific types of immune cells - eosinophils and neutophils - were reduced by 31% and 42% after 4 weeks of treatment with Roflumilast.
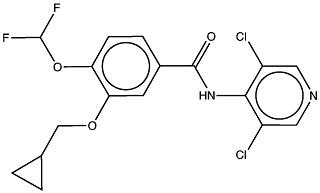
Roflumilast ( IUPAC: 3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide InChI: 1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) SMILES: FC(F)Oc1ccc(cc1OCC2CC2)C(=O)Nc3c(Cl)cncc3Cl Chemspider:395793 ChEMBL:193240) is a synthetic small molecule drug containing no chiral centers. It has a molecular weight of 403.2 Da and calculated LogP of 4.4. Roflumilast has 4 hydrogen bond acceptors and 1 hydrogen bond donor and therefore fully complies with Lipinski's rule of five.
The structure of a number of phosphodiesterase enzymes are known, including a number of PDE4 isoforms, a typical complex of PDE4D with an inhibitor is PDBe:1y2k
Roflumilast is administered once daily as an oral tablet containing 500 ug of active ingredient (equivalent to 1.2 umol).
The full prescribing information can be found here.
Roflumilast was approved by the European commission in 2010 and is marketed in Europe as Daxas. In the US, Roflumilast will be marketed by Forest Pharmaceuticals under the trade name Daliresp (product website).