New Drug Approvals 2013 - Pt. II - Mipomersen (KynamroTM)
On January 29st, the FDA approved Mipomersen (Tradename: Kynamro; Research Code: ISIS-310312), an oligonucleotide inhibitor of apolipoprotein B-100 (apo B-100) synthesis, indicated as an adjunct to lipid-lowering medications and diet to reduce low density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apo B), total cholesterol (TC), and non-high density lipoprotein-cholesterol (non HDL-C) in patients with homozygous familial hypercholesterolemia (HoFH).
Familial hypercholesterolemia is a genetic disorder, characterised by high levels of cholesterol rich low-density lipoproteins (LDL-C) in the blood. This genetic condition is generally attributed to a faulty mutation in the LDL receptor (LDLR) gene, which mediates the endocytosis of LDL-C.
Mipomersen is the first antisense oligonucleotide that targets messenger RNA (mRNA) enconding apolipoprotein B-100 (Apo B-100), the principal apolipoprotein of LDL and its metabolic precursor, very low density lipoprotein (VLDL). Mipomersen forms a duplex with the target mRNA, causing the mRNA to be cleaved by RNase H and therefore unable to be translated to apoB-100. Hepatic apoB mRNA silencing gives rise to reductions in hepatic apoB, total cholesterol and LDL-C levels in the serum (PMID: 23226021).
The binding site for mipomersen lies within the coding region of the apo B mRNA at the position 3249-3268 relative to the published sequence GenBank accession number NM_000384.1.
Mipomersen, like other antisense oligonucleotides, belongs to the -rsen USAN/INN stem group. Several members of this class are currently in clinical trials like Alicaforsen (Isis, phase III) for the treatment of inflammatory bowel disease and Oblimersen (Genta, phase II) for cancer therapy.
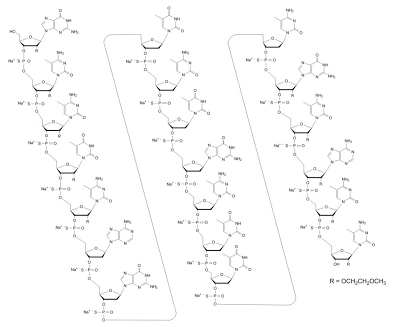
Mipomersen (IUPAC Name: 2'-O-(2-methoxyethyl)-P-thioguanylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl- P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')- 2'-O-(2-methoxyethyl)-5-methyl-P-thiouridylyl-(3'→5')-2'-O-(2-methoxyethyl)-5- methyl-P-thiocytidylyl-(3'→5')-2'-deoxy-P-thioadenylyl-(3'→5')-2'-deoxy-P- thioguanylyl-(3'→5')P-thiothymidylyl-(3'→5')-2'-deoxy-5-methyl-P-thiocytidylyl- (3'→5')-P-thiothymidylyl-(3'→5')-2'-deoxy-P-thioguanylyl-(3'→5')-2'-deoxy-5- methyl-P-thiocytidylyl-(3'→5')-P-thiothymidylyl-(3'→5')-P-thiothymidylyl- (3'→5')-2'-deoxy-5-methyl-P-thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-P- thioguanylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P-thiocytidylyl-(3'→5')-2'- O-(2-methoxyethyl)-P-thioadenylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methyl-P- thiocytidylyl-(3'→5')-2'-O-(2-methoxyethyl)-5-methylcytidine nonadecasodium salt; Canonical smiles: COCCO[C@H]1[C@@H](O)[C@H](COP(=O)(O)S[C@H]2[C@H](COP(=O)(O)S[C@H]3[C@H](COP(=O)(O)S[C@H]4[C@H](COP(=O)(O)S[C@@H]5[C@@H](COP(=O)(O)S[C@H]6C[C@@H](O[C@@H]6COP(=O)(O)S[C@H]7C[C@@H](O[C@@H]7COP(=O)(O)S[C@H]8C[C@@H](O[C@@H]8COP(=O)(O)S[C@H]9C[C@@H](O[C@@H]9COP(=O)(O)S[C@H]%10C[C@@H](O[C@@H]%10COP(=O)(O)S[C@H]%11C[C@@H](O[C@@H]%11COP(=O)(O)S[C@H]%12C[C@@H](O[C@@H]%12COP(=O)(O)S[C@H]%13C[C@@H](O[C@@H]%13COP(=O)(O)S[C@H]%14C[C@@H](O[C@@H]%14COP(=O)(O)S[C@H]%15C[C@@H](O[C@@H]%15COP(=O)(O)S[C@@H]%16[C@@H](COP(=O)(O)S[C@@H]%17[C@@H](COP(=O)(O)S[C@@H]%18[C@@H](COP(=O)(O)S[C@@H]%19[C@@H](COP(=O)(O)S[C@@H]%20[C@@H](CO)O[C@H]([C@@H]%20OCCOC)n%21cnc%22C(=O)NC(=Nc%21%22)N)O[C@H]([C@@H]%19OCCOC)N%23C=C(C)C(=NC%23=O)N)O[C@H]([C@@H]%18OCCOC)N%24C=C(C)C(=NC%24=O)N)O[C@H]([C@@H]%17OCCOC)N%25C=C(C)C(=O)NC%25=O)O[C@H]([C@@H]%16OCCOC)N%26C=C(C)C(=NC%26=O)N)n%27cnc%28c(N)ncnc%27%28)n%29cnc%30C(=O)NC(=Nc%29%30)N)N%31C=C(C)C(=O)NC%31=O)N%32C=C(C)C(=NC%32=O)N)N%33C=C(C)C(=O)NC%33=O)n%34cnc%35C(=O)NC(=Nc%34%35)N)N%36C=C(C)C(=NC%36=O)N)N%37C=C(C)C(=O)NC%37=O)N%38C=C(C)C(=O)NC%38=O)N%39C=C(C)C(=NC%39=O)N)O[C@H]([C@@H]5OCCOC)n%40cnc%41C(=O)NC(=Nc%40%41)N)O[C@@H]([C@H]4OCCOC)N%42C=C(C)C(=NC%42=O)N)O[C@@H]([C@H]3OCCOC)n%43cnc%44c(N)ncnc%43%44)O[C@@H]([C@H]2OCCOC)N%45C=C(C)C(=NC%45=O)N)O[C@@H]1N%46C=C(C)C(=NC%46=O)N; ChEMBL: CHEMBL1208153; Standard InChI Key: TZRFSLHOCZEXCC-PBNBMMCMSA-N) is a synthetic second-generation 20-base phosphorothioate antisense oligonucleotide, with a molecular weight of 7594.9 Da and the following sequence:
5'-GMeCMeCMeUMeCAGTMeCTGMeCTTMeCGMeCAMeCMeC-3'
where the underlined residues are 2′-O-(2-methoxyethyl) nucleosides, and the remaining are 2′-deoxynucleosides. Substitution at the 5-position of the cytosine (C) and uracil (U) bases with a methyl group is indicated by Me.
Mipomersen is available as an aqueous solution for subcutaneous injection, and the recommended weekly dose is a single-use pre-filled syringe containing 1 mL of a 200 mg/mL solution. Following subcutaneous injection, peak concentrations of mipomersen are typically reached in 3 to 4 hours. The estimated plasma bioavailability of mipomersen following subcutaneous administration over a dose range of 50 mg to 400 mg, relative to intravenous administration, ranged from 54% to 78%. Mipomersen is highly bound to human plasma proteins (≥ 90%).
Mipomersen is metabolised in tissues by endonucleases to form shorter oligonucleotides that are then substrates for additional metabolism by exonucleases, and finally excreted in urine. The elimination half-life (t1/2) for mipomersen is approximately 1 to 2 months.
Mipomersen is metabolised in tissues by endonucleases to form shorter oligonucleotides that are then substrates for additional metabolism by exonucleases, and finally excreted in urine. The elimination half-life (t1/2) for mipomersen is approximately 1 to 2 months.
Mipomersen has been approved with a black box warning due to an increase in transaminases (alanine aminotransferase [ALT] and/or aspartate aminotransferase [AST]) levels, and hepatic fat content (steatosis) after exposure to the drug.
The license holder for KynamroTM is Genzyme Corporation, and the full prescribing information can be found here.