

Second onto market this year is Febuxostat, approved on the 13th of February. Febuxostat is a
xanthine oxidase inhibitor used for the treatment of hyperuricemia (gout), and competes against well established agents such as Allopurinol (Allopurinol was approved in 1964 for the treatment of gout). Febuxostat is a small molecule drug (Molecular Weight of 316.4 g.mol-1) is fully Rule-Of-Five compliant, is essentially insoluble in water, and has good oral absorption (>49% bioavailable). Febuxostat plasma half-life of 5 to 8 hours, a volume of distribution of 50L, high plasma protein binding at 99.2% (unsurprising given the lipophilic acid nature of the molecule). Febuxostat is extensively metabolised, both by the UGT system, producing glucoronidated metabolites, and also by a range of complex CYP mediated transformations (metabolites of metabolites....). Some of the CYP mediated metabolites involve hydroxylation of the butyl sidechain, these themselves are pharmacologically active against the target. Roughly half the dose is cleared renally, and the other half in the faeces. Typical daily dosing is 40mg (or ~126µmol), or 80mg if insufficient efficacy is observed. The full prescribing information is
here.
The structure (2-[3-cyano-
4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid) contains a nitrile group, and a free carboxylic acid group (which will make the drug negatively charged under physiologic conditions and dominate its physical chemistry). The molecule is quite rigid and comparatively planar (due to the presence of sp2 hybridized atoms adjacent to the core phenyl and thiazole rings). A nitrile is quite unusual in drugs due to its comparative susceptibility to nucleophilic attack, and also metabolic stability - nitriles can be hydrolysed to carboxylic acids.
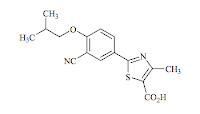
Febuxostat canonical SMILES: CC1=C(SC(=N1)C2=CC(=C(C=C2)OCC(C)C)C#N)C(=O)O
Febuxostat InChI: InChI=1S/C16H16N2O3S/c1-9(2)8-21-13-5-4-11(6-12(13)7-17)15-18-10(3)14(22-15)16(19)20/h4-6,9H,8H2,1-3H3,(H,19,20)
Febuxostat InChIKey: BQSJTQLCZDPROO-UHFFFAOYSA-N
Febuxostat CAS registry: 144060-53-7
Febuxostat Chemdraw: Febuxostat.cdx
The manufacturer of Febuxostat is Takeda Pharmaceuticals and the product website is www.uloric.com


