New Drug Approvals - Pt. VIII - Iloperidone (Fanapt)
Iloperidone elimination is mainly through CYP dependent hepatic metabolism (specifically CYP3A4 and CYP2D6); some of these metabolites are themselves pharmacologically active. CYP2D6 is an example of a genetically variable gene, with a fraction of the population having a form of the gene that is significantly less active - so called 'slow metabolizers', this generally leads to variability of drug response. Iloperidone has an apparent clearance of 47-102 L/hour, with the bulk of the clearance being renal (i.e.. the drug is excreted in the urine). The recommended dosage is 12 to 24 mg/day administrated twice daily (equivalent to ca. 28-56uM). Like many drugs of this class, there are a broad range of potential adverse events, including a propensity for the compound to increase QTc interval. The full prescribing information can be found here.
Iloperidone has a boxed warning (colloquially known as a 'black box').
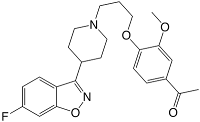
Iloperidone belongs to the chemical class of piperidinyl-benzisoxazole derivatives - the piperidine in the six membered ring containing the nitrogen in the middle of the molecule, while the benzisoxazole is the fused five-six dual ring structure at the bottom left. Its structure 4'-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidino]propoxy]-3'-methoxyacetophenone contains a tertiary amine which makes the molecule basic, but otherwise the molecule is largely lipophilic in character. A relatively unusual chemical feature for a drug is the presence of the aryl-ketone group.
Iloperidone canonical SMILES: O=C(c4ccc(OCCCN3CCC(c2noc1cc(F)ccc12)CC3)c(OC)c4)C Iloperidone InChI: InChI=1/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-2 7-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17 H,3,8-13H2,1-2H3 Iloperidone InChIKey: XMXHEBAFVSFQEX-UHFFFAOYAT Iloperidone CAS registry: 133454-47-4 Iloperidone ChemDraw: Iloperidone.cdx
The license holder for Iloperidone is Vanda Pharmaceuticals and the product website is www.fanapt.com