-
New Drug Approvals - Pt. VI - Artemether/Lumefantrine (Coartem)
Artemether: 
Lumefantrine: 
Next in our series of posts on 2009 new drug approvals is a mixture of Artemether (USAN) - a methyl ether derivative of artemisinin, and Lumefantrine (USAN) - a racemic mixture of a synthetic racemic fluorene derivative formerly known as benflumetol. The combination of these two drugs in one product was approved on April 7th. The mixture is marketed under the trade name Coartem, for the treatment of acute, uncomplicated malaria infections due to Plasmodium falciparum, the causative parasite of the most aggressive form of the disease. Artemether and Lumefantrine are both small molecule drugs (molecular weights of 298.4 g.mol-1 and 528.9 g.mol-1, respectively), lipophilic, and are both essentially insoluble in water. Artemether is fully Rule-of-Five compliant, whereas Lumefantrine is not.
Artemether and Lumefantrine have increased oral absorption when administrated with food and display high plasma protein binding of 95.4% and 99.7%, respectively. Artemether and Lumefantrine are both metabolized mainly by CYP3A4 are therefore have many drug-drug interactions with substrates, inhibitors and inducers of CYP3A4. Artemether is extensively metabolized to Dihydroartemisin (DHA), which then undergoes rapid clearance. Therefore, Artemether, which has an elimination half-life of 2 hours, is administrated with Lumefantrine, which has a much longer and pharmacologically complementary terminal half-life of 3-6 days and therefore the two drugs act synergistically against Plasmodium.
Recommended dosage and full prescribing information can be found here.
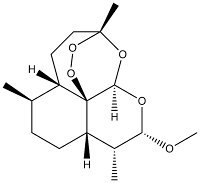
The Artemether structure is (3R,5aS,6R,8aS,9R,10S,12R,12aR)-decahydro-10-methoxy-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepine. The molecule is extremely rigid, with very few rotational bonds. An important chemical feature and unique in drugs is the presence of a peroxide bond (the O-O single bond), which is essential for the antimalarial activity.
Artemether canonical SMILES: C[C@@H]1CC[C@@H]3C42OO[C@](C)(CC[C@@H]12)O[C@H]4O[C@H](OC)[C@@H]3C Artemether InChI: InChI=1/C16H26O5/c1-9-5-6-12-10(2)13(17-4)18-14-16(12)11(9)7-8-15 (3,19-14)20-21-16/h9-14H,5-8H2,1-4H3/t9-,10-,11+,12+,13+,14-,15-, 16?/m1/s1 Artemether InChIKey: SXYIRMFQILZOAM-LJXHFVHTBB Artemether CAS registry: 71963-77-4 Artemether ChemDraw: Artemether.cdx
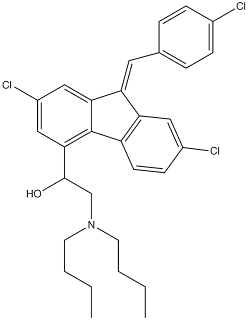
The Lumefantrine structure is (±)-2-dibutylamino-1-[2,7-dichloro-9-(4-chlorobenzylidene)-9H-fluorene-4-yl]ethanol. The molecule is an amino-alcohol, with a tertiary amine (which can be protonated and therefore Lumefantrine is normally positively charged). It also contains a racemic stereocenter at the carbon to which the hydroxyl group is attached. Since the drug is racemic, the stereoisomers have different pharmacological activity, and differing metabolic properties. The remainder of the molecule is quite rigid, with two chlorophenyls fused together by a five-membered ring with a substituted benzylidene group.
Lumefantrine canonical SMILES: Clc1ccc(cc1)\C=C3/c4c(c2c(cc(Cl)cc23)C(O)CN(CCCC)CCCC)ccc(Cl)c4 Lumefantrine InChI: InChI=1/C30H32Cl3NO/c1-3-5-13-34(14-6-4-2)19-29(35)28-18-23(33)17 -27-25(15-20-7-9-21(31)10-8-20)26-16-22(32)11-12-24(26)30(27)28/h 7-12,15-18,29,35H,3-6,13-14,19H2,1-2H3/b25-15+ Lumefantrine InChIKey: DYLGFOYVTXJFJP-MFKUBSTIBV Lumefantrine CAS registry: 82186-77-4 Lumefantrine ChemDraw: Lumefantrine.cdx
The manufacturer of Artemether/Lumefantrine combination is Novartis and the product website is www.coartem.com
-
New Drug Approvals - Pt. V - Everolimus (Afinitor)
Also approved this year, on March 30th 2009, is Everolimus (USAN). Everolimus is an inhibitor of mTOR (mammalian target of rapamycin), a serine-threonine kinase, and is indicated for the treatment of advanced renal cell carcinoma after failure of treatment with Sunitinib or Sorafenib. Sunitinib and Sorafenib are both orally dosed small molecule inhibitors of protein kinases. Everolimus is also marketed under the trade name (although not currently in the US) as Certican, which is used for immunosuppression in transplant therapy. Everolimus (previously known by the research code RAD-001) is a relatively large 'small molecule' drug (Molecular Weight of 958.2 g.mol-1), lipophilic, orally absorbed and has a low plasma binding of ~74%. Everolimus is primarily metabolized
CYP3A4 routes, with known metabolites being essentially inactive as mTor inhibitors, these are largely excreted in the feces. Everolimus has a long mean elimination half-life of ~30 hours. Typical dosage is 10 mg (equivalent to ca. 10.4 umol) once a day. The full prescribing information can be found here. The structure (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18- dihydroxy-12-{(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-methylethyl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone) contains a macrolide ring (a large macrocyclic ester), often a characteristic of natural products. In fact, with the complex size, high number of defined stereocenters Everolimus is very 'natural product like'. of striking functional group interest is the presence of the unusual alpha-keto amide functionality (the two adjacent carbonyls and the amide from the six membered piperidine ring - this has unusual conformational and reactivity properties, and is associated with the conformation required for cis- to trans-isomerisation for a proline containing peptide.
Everolimus is chemcially very similar to the natural product drug Rapamycin (in fact one chemical name for Everolimus is 42-O-(2-hydroxyethyl)-Rapamycin). Confusingly Rapamycin is also known by the USAN Sirolimus. Sirolimus was originally identified as an active component of a soil isolate from Easter Island, eventually the source of this molecule was found to be the bacteria Streptomyces hygroscopicus. A further member of the family is the drug Tacrolimus (USAN) (also know by the research code FK-506) which was isolated from a Japanese soil sample, and is made by the bacteria Streptomyces tsukubaensis. Rather excitingly drugs of this class have been very recently shown to extend the lifespan of mice (click here for a pdf of the Nature paper, and here for further media coverage). Of course, due to the other functions of Rapamycin (suppression of the immune system) drugs of this class may not actually be that useful for the extension of life.
Everolimus canonical SMILES: O=C2[C@H](C)C[C@@H](\C=C\C=C/C=C(\C)[C@@H](OC)C[C@H]4O[C@@](O)(C(=O)C(=O)N3[C@H](C(=O)O[C@H]([C@H](C)C[C@@H]1CC[C@@H](OCCO)[C@H](OC)C1)CC(=O)[C@@H](/C=C(/C)[C@@H](O)[C@H]2OC)C)CCCC3)[C@@H](CC4)C)C Everolimus InChI: InChI=1/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19- 38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35( 4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6) 48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43 -46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11-,16-12+,33- 17+,37-27-/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,4 9+,53-/m1/s1 Everolimus InChIKey: HKVAMNSJSFKALM-CNPAPGRKBU Everolimus CAS registry: 159351-69-6 Everolimus ChemDraw: Everolimus.cdx
The manufacturer of Everolimus is Novartis and the product website is www.afinitor.com.
-
chEMBL Database Infrastructure Poll
A reminder on the poll we are running to help us plan for raw data distribution of the chEMBL data. We will close the poll on Friday 19th June.
Results are non-binding, and are to assist us in our technical planning.....
-
MipTec2009 - October 13th-15th, Basel, 2009
We are going to speak at the forthcoming MipTec meeting in Basel - this is a free registration meeting, so it is pretty unusual (and good!). We will speak on bioisostere discovery from our chEMBL databases - probably in the area of peptide drugs and peptide bioisosteres.
The meeting website is here....
As you may have noticed, today is a catch up with things-to-do-day, and also we are celebrating some of the kings (and maybe later, queens) of british comedy in the the blog pictures.
-
Browsers and OS's for Chemblog access
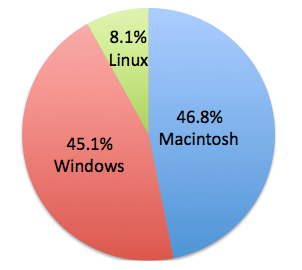
So we've had well in excess of 10,000 accesses to the chembl-og so far, and I thought it might be time to do a little bit of analysis of the accesses do far in terms of OS, and browsers, java-support and so forth. All the sort of nice details provided by google analytics. Of course, the sample here may not be representative of the eventual users of the group's data, but I found the results interesting, and they will be factored into our interface testing processes. As an aside, all numbers are for 'Visits'.
Operating Systems
Browsers
85% of browsers have java enabled. 98.4% have 24 bit (or higher) colour. 92% have 1024x768 (or higher) screen resolution, 81.8% have 1200x800 (or higher) screen resolution.
-
What database systems should we support for chEMBL data?
Here is a poll asking for input on the primary (and potentially other) database systems for distribution of the chEMBL data.
UPDATE: Poll is now closed.
-
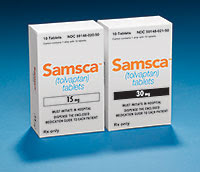
New Drug Approvals - Pt. IV - Tolvaptan (Samsca)
 Latest through the gates is Tolvaptan (trade name Samsca), approved on May 21st 2009. Tolvaptan is a first-in-class oral vasopressin receptor antagonist used for the treatment of hyponatremia (low plasma sodium levels). Tolvaptan (previously known as the research code OPC-41,061) is selective for the Vasopressin V2 receptor isoform (reportedly 29 fold selective over the V1a receptor isoform. There are a number of -vaptans (the USAN stem for vasopressin antagonists) of varying selectivity profiles in clinical trials (including Conivaptan, Relcovaptan, SSR-149,415, Lixivaptan, Mozavaptan, and Satavaptan) for a variety of differing indications; but Tolvaptan is the first to be approved in the US.
Latest through the gates is Tolvaptan (trade name Samsca), approved on May 21st 2009. Tolvaptan is a first-in-class oral vasopressin receptor antagonist used for the treatment of hyponatremia (low plasma sodium levels). Tolvaptan (previously known as the research code OPC-41,061) is selective for the Vasopressin V2 receptor isoform (reportedly 29 fold selective over the V1a receptor isoform. There are a number of -vaptans (the USAN stem for vasopressin antagonists) of varying selectivity profiles in clinical trials (including Conivaptan, Relcovaptan, SSR-149,415, Lixivaptan, Mozavaptan, and Satavaptan) for a variety of differing indications; but Tolvaptan is the first to be approved in the US.
Tolvaptan has a boxed warning (colloquially known as a 'black box').
Tolvaptan is a racemic small molecule drug (Molecular Weight of 448.9 g.mol-1) and is a lipophilic drug, is essentially insoluble in water, and has fair oral absorption (>40% bioavailable). Tolvaptan hsa a plasma half-life of around 12 hours, a volume of distribution of 3L/kg, and has high plasma protein binding at 99%. Tolvaptan is extensively metabolised by 3A4, and therefore has many concommitent interactions with 3A4 substrates, inhibitors and inducers. Reportedly, Tolvaptan metabolites show no appreciable pharmacology against the vasopressin receptors. Interestingly, Tolvaptan is a racemic drug, with the two enantiomers showing differing PK/PD. Typical daily dosing is 30mg (or ~67µmol), or 60mg if insufficient efficacy is observed. The full prescribing information is here.
The structure (±)-4'-[(7-chloro-2,3,4,5-tetrahydro-5-hydroxy-1H-1-benzazepin-1-yl) carbony1]- o-tolu-m-toluidide. The molecule is rigid, with very few freely rotatable bonds, and comparatively planar (due to the presence of sp2 hybridized atoms adjacent to the core phenyl ring). The racemic stereocenter is at the hydroxyl on the benzazepine (a benzazepine is a seven-membered nitrogen containing heterocycle (an azepine) fused to benzene ring). The neutral, largely lipophilic and aromatic nature of Tolvaptan is consistent with the reported 3A4 avidity. Finally, another feature of note is the presence of an aniline in the molecule (an aniline is an aromatic amine, here in Tolvaptan it is masked as an amide); anilines have a reputation for being associated with unwanted toxicities with drugs, although this is by no-way a hard and fast rule!
Tolvaptan canonical SMILES: CC1=CC=CC=C1C(=O)NC2=CC(=C(C=C2)C(=O)N3CCCC(C4=C3C=CC(=C4)Cl)O)C Tolvaptan InChI: InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14- 19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15, 24,30H,5,8,13H2,1-2H3,(H,28,31) Tolvaptan InChIKey: GYHCTFXIZSNGJT-UHFFFAOYSA-N Tolvaptan CAS registry: 150683-30-0 Tolvaptan Chemdraw: Tolvaptan.cdx
The license holder for Tolvaptan is Otsuka Pharmaceuticals and the product website is www.samsca.com
-
Industry Partner Druggability Workshop
 On Friday July 10th we plan to hold a further Druggability Workshop for the EMBL-EBI Industry Partners. If anyone is interested in participating in this highly interactive and participatory workshop AND also interested in joining the Industry Partner program; please contact me.....
On Friday July 10th we plan to hold a further Druggability Workshop for the EMBL-EBI Industry Partners. If anyone is interested in participating in this highly interactive and participatory workshop AND also interested in joining the Industry Partner program; please contact me.....