New Drug Approvals 2011 - Pt. XIX Belatacept (NulojixTM)
On June 15th 2011, the FDA has approved Belatacept (trade name: Nulojix; Research Code: BMS-224818), a selective T-cell (lymphocyte) costimulation blocker indicated for phophylaxis of organ rejection in adult patients receiving a kidney transplant. Belatacept is approved for use in combination with other immunosuppressants, specifically basiliximab, mycophenolate mofetil and corticosteroids.
Belatacept is a potent antagonist that inhibits T-lymphocyte activation by binding to the B7-ligands, namely CD80 (Uniprot: P33681; Pfam: PF08205, PF07686) and CD86 (Uniprot: P42081; Pfam: PF07686), present on antigen-presenting cells, and thereby blocking interaction with CD28 (Uniprot: P10747; Pfam: PF07686), the receptor of these two ligands. This interaction provides a costimulary signal necessary for full activation of T-lymphocytes. Activated T-cells are the predominant mediators of immunologic rejection. In vitro, Belatacept inhibits T-cell proliferation and the cytokines interleukin-2, interferon-γ, interleukin-4 and TNF-α.
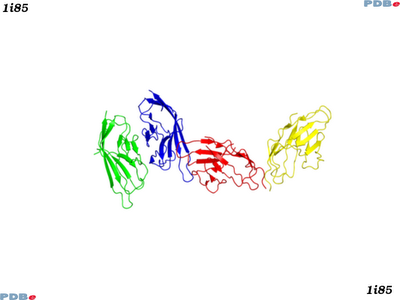
There are some protein structures known for the B7-ligands, CD80 and CD86. Here are two typical entries for CD80 (PDBe:1i8l) and CD86 (PDBe:1i85) in complex with CTLA-4.

 Belatacept is derived from Abatacept (trade name: Orencia; approved in 2005 for the treatment of rheumatoid arthritis, ChEMBLID: CHEMBL1201823), a soluble fusion protein that consists of the extracellular domain of the human cytotoxic T-lymphocyte antigen-4 (CTLA-4; Uniprot: P16410; Pfam: PF07686), linked to a modified Fc (hinge-CH2-CH3 domains) portion of human immunoglobulin G1 (CTLA4-Ig). CTLA-4 is similar to the T-cell costimulatory protein CD28, and both molecules bind to CD80 and CD86 on antigen-presenting cells. However, CTLA-4 transmits an inhibitory signal to T-cells, whereas CD28 transmits a stimulatory signal. Although Abatacept binds to the B-7 ligands with higher affinity when compared with CD28, it has never reached the market as an organ transplantation therapy due to the fact that it does not completely and equally block of the costimulation pathway (the difference in antagonistic effect to CD80 compared with CD86 is a 100-fold decrease in affinity to the CD86). Given this, Belatacept was developed by altering two amino acids in the B-7 ligand-binding portion of the Abatacept molecule (a leucine and an alanine were replaced by a glutamic acid and a tyrosine, respectively). These modifications have resulted in a 4-fold increase in binding affinity to the CD86 and a 2-fold increase in CD80 binding affinity in comparison to Abatacept. Also, it has been shown that, in vitro, this increase in binding affinity to the B-7 ligands resulted in a 10-fold increase in inhibiting T-cell activation when compared with Abatacept.
Belatacept is derived from Abatacept (trade name: Orencia; approved in 2005 for the treatment of rheumatoid arthritis, ChEMBLID: CHEMBL1201823), a soluble fusion protein that consists of the extracellular domain of the human cytotoxic T-lymphocyte antigen-4 (CTLA-4; Uniprot: P16410; Pfam: PF07686), linked to a modified Fc (hinge-CH2-CH3 domains) portion of human immunoglobulin G1 (CTLA4-Ig). CTLA-4 is similar to the T-cell costimulatory protein CD28, and both molecules bind to CD80 and CD86 on antigen-presenting cells. However, CTLA-4 transmits an inhibitory signal to T-cells, whereas CD28 transmits a stimulatory signal. Although Abatacept binds to the B-7 ligands with higher affinity when compared with CD28, it has never reached the market as an organ transplantation therapy due to the fact that it does not completely and equally block of the costimulation pathway (the difference in antagonistic effect to CD80 compared with CD86 is a 100-fold decrease in affinity to the CD86). Given this, Belatacept was developed by altering two amino acids in the B-7 ligand-binding portion of the Abatacept molecule (a leucine and an alanine were replaced by a glutamic acid and a tyrosine, respectively). These modifications have resulted in a 4-fold increase in binding affinity to the CD86 and a 2-fold increase in CD80 binding affinity in comparison to Abatacept. Also, it has been shown that, in vitro, this increase in binding affinity to the B-7 ligands resulted in a 10-fold increase in inhibiting T-cell activation when compared with Abatacept. >Belatacept MHVAQPAVVLASSRGIASFVCEYASPGKYTEVRVTVLRQADSQVTEVCAATYMMGNELTFLDDSICTGTSSGNQVNLTIQ GLRAMDTGLYICKVELMYPPPYYEGIGNGTQIYVIDPEPCPDSDQEPKSSDKTHTSPPSPAPELLGGSSVFLFPPKPKDT LMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPA PIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKL TVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGKOther immunosuppressive therapies to treat transplant rejection are available on the market and these include calcineurin inhibitors, such as Tacrolimus (ChEMBLID: CHEMBL1237096), mTOR inhibitors, such as Everolimus (ChEMBLID: CHEMBL1201755), anti-proliferatives, such as Mycophenolic acid (ChEMBLID: CHEMBL866), corticosteroids, such as Hydrocortisone (ChEMBLID: CHEMBL389621) and antibodies, such as Basiliximab (ChEMBLID: CHEMBL1201439) and Rituximab (ChEMBLID: CHEMBL1201576).
Belatacept recommended dosage is a 10 mg/kg intravenous infusion on days 1 (day of transplantation) and 5, end of weeks 2, 4, 8, and 12 after transplantation in the initial phase, followed by a maintenance phase of 5 mg/kg at the end of week 16 after transplantation and every 4 weeks thereafter. The molecular weight of Belatacept is approximately 90 kDa. After a 10 mg/kg intravenous infusion at week 12, Belatacept has a volume of distribution (Vd) of 0.11 L/kg, a systemic clearance (CL) of 0.49 mL/h/kg and a terminal half-life (t1/2) is 9.8 days. The full prescribing information can be found here.
The license holder is Bristol-Myers Squibb Company and the product website is www.nulojix.com.

