-
New Drug Approvals - Pt. XV - Asenapine (Saphris)
On the 14th August 2009 Asenapine (tradename Saphris) was approved for the acute treatment of schizophrenia in adults and acute treatment of manic or mixed episodes associated with bipolar I disorder with or without psychotic features in adults. This class of psychiatric diseases are complex and carry a significant economic healthcare burden; approximately 24 million people worldwide are believed to suffer from schizophrenia, while ca. 67 million people are thought to suffer from bipolar I disorder. Asenapine (previously known by the research code Org-5222) is the one of a large class of drugs aimed at treating such diseases, and shows the typical broad spectrum of against a variety of receptor targets and a complicated mechanism of action, although such drugs are thought to primarily act through antagonism of D2 and 5HT2A receptors. To give some idea of the promiscuity (or polypharmacology) of Asenapine at various aminergic GPCRs, reported pKis are 5HT1A 8.6, 5HT1B 8.4, 5HT2A 10.2, 5HT2B 9.8, 5HT2C 10.5, 5HT5A 8.8, 5HT6 9.5, 5HT7 9.9, alpha1 8.9, alpha2A 8.9, alpha2B 9.5, alpha2C 8.9, D1 8.9, D2 8.9, D3 9.4, D4 9.0, H1 9.0, and H2 8.2. Asenapine has had quite a complex development and commercial history, as web searches will readily show.
Asenapine is a small molecule drug (Molecular Weight of 285.8 g.mol-1 for Asenapine itself, and 401.84 g.mol-1 for the Asenapine Malate dosed ingredient). Asenapine is reasonably absorbed with an absolute bioavailability of 35% for sublingual dosing - for oral dosing (i.e. the drug makes it into the stomach and bowel absorption is far lower at ca. 2%. (This route of absorption also is really 'topical', even through thr drug is orally dosed, hence the topical icon above - confusing -eh?). Asenapine has high plasma protein binding (~95%), and a volume of distribution of 20-25 L.kg-1). It also has a high clearance - 52 L.hr-1. Asenapine is primarily metabolized by oxidative metabolism by CYP1A2 and also by direct glucoronidation by UGT1A4, and is cleared by both renal and hepatic routes in approximately similar proportions. It has a an elimination half-life of ca. 24 hours and due to this long half-life, steady-state plasma concentrations are reached after approximately three days. Recommended dosage is one tablet of 5 mg twice daily (equivalent to ca. 24.9 µmol). The full prescribing information can be found here.
Asenapine has a boxed warning.
The Asenapine structure is (3aRS,12bRS)-5-Chloro-2-methyl-2,3,3a,12b-tetrahydro-1Hdibenzo[2,3:6,7]oxepino[4,5-c]pyrrole - and more broadly is member of a set of molecules called dibenzo-oxepino pyrroles. It is fully rule-of-five-compliant. The structure is reasonably unremarkable in terms of its functional group content - the only distinctive feature is the basic amine, but other than being primarily flat, highly rigid and planar, it is difficult to highlight anything more specific.
Asenapine canonical SMILES: CN1CC2C(C1)C3=C(C=CC(=C3)Cl)OC4=CC=CC=C24 Asenapine InChI: InChI=1S/C17H16ClNO/c1-19-9-14-12-4-2-3-5-16(12)20-17-7-6-11(18)8-13(17) 15(14)10-19/h2-8,14-15H,9-10H2,1H3/t14-,15-/m1/s1 Asenapine InChIKey: VSWBSWWIRNCQIJ-HUUCEWRRSA-N Asenapine CAS registry: 65576-45-6 Asenapine ChemDraw: Asenapine.cdx
The license holder for Asenapine is Schering Plough and the product website is www.saphris.com.
-
New Drug Approvals - Pt. XIV - Pitavastatin (Livalo)

The latest FDA approval is Pitavastatin (trade name Livalo), approved on August 3rd. Pitavastatin is an HMG-CoA reductase inhibitor, indicated for the primary treatment of hypercholesterolemia (elevated levels of cholesterol in the blood) on patients unable to sufficiently lower their cholesterol levels by diet and exercise. Hypercholesterolemia is a very widespread and leads to serious cardiovascular disease in affluent and increasingly in developing societies.
Pitavastatin (also known by the research code NKS-104) has been available in Japan since 2003 and is now the sixth statin to reach the U.S. market, after Lovastatin (trade name Mecavor), Pravastatin (trade name Pravachol), Fluvastatin (trade name Lescol), Atorvastatin (trade name Lipitor) and Rosuvastatin (trade name Crestor). All of these drugs are derived from the natural product Mevastatin from the fungus Penicillium citrinum. Like the other statins, Pitavastatin lowers the cholesterol levels by competitively inhibiting HMG-CoA reductase, which is the rate-limiting enzyme of the mevalonate pathway of cholesterol synthesis. Inhibition of this enzyme in the liver results in decreased cholesterol synthesis as well as increased synthesis of low-density lipoprotein (LDL) receptors, resulting in a greater low-density lipoprotein cholesterol (LDL-C) clearance and overall reduction of cholesterol from the bloodstream. However, even in this relatively well studied area of biology, there are still many questions to be answered over cholesterol flux and pathogenesis.
Pitavastatin is small-molecule drug (Molecular Weight of 421.5 g.mol-1), and is fully Rule-of-Five compliant. Pitavastatin has a high bioavailability of 51%, and high protein plasma binding (ppb) of 99%), along with volume of distribution of 148 L and a half-life of 11 hours. Metabolism is primarily by glucuronidation (see below) but there is minor oxidative processing by CYP2C9 (and to a lesser by CYP2C8). The major metabolite isolated from human plasma is the lactone is formed via an ester-type pitavastatin glucuronide conjugate catalysed by UGT1A3 and UGT2B7. Excretion is primarily through feces (79% of dose). Typical dosage is of a single 2 mg tablet once a day (equivalent to a daily dose of 4.8 umol).
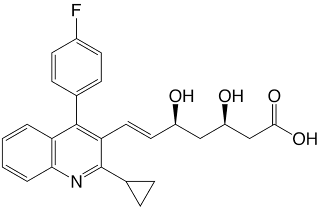
The structure (3R,5S,6E)-7-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-3,5-dihydroxyhept-6-enoic acid contains a diol group with two defined stereocenters and a free carboxylic acid group (which mimic the mevalonic acid substrate of the enzyme). The acid group will make the drug negatively charged under physiological conditions and dominate its physical chemistry), common features among many synthetic statins. The remainder of the molecule is largely lipophilic and rigid.
Pitavastatin canonical SMILES: O=C(O)CC(O)CC(O)/C=C/c1c(c3ccccc3nc1C2CC2)c4ccc(F)cc4
Pitavastatin InChI: InChI=1/C25H24FNO4/c26-17-9-7-15(8-10-17)24-20-3-1-2-4-22(20)27-2 5(16-5-6-16)21(24)12-11-18(28)13-19(29)14-23(30)31/h1-4,7-12,16,1 8-19,28-29H,5-6,13-14H2,(H,30,31)/b12-11+
Pitavastatin InChIKey: VGYFMXBACGZSIL-VAWYXSNFBA
Pitavastatin CAS registry: 147511-69-1
Pitavastatin ChemDraw: Pitavastatin.cdx
The license holder is Kowa Pharmaceuticals and the product website is www.livalo.com -
What Do Modern Drugs Do?
A quick follow up to the modern drugs post of a few days ago. Here is an overview of the WHO ATC classification of recent drugs (it is not quite the same dataset as before (I have extended it back a year, and not all modern drugs have been assigned ATC codes yet) but it gives an idea of what physiological systems more modern drugs act on.
It is of note that in excess of half of all modern drugs are targeted at cancer, the nervous system, and anti-infective disease.
-
Properties of Modern Drugs
 So, I have a paper review to finish off, and then assess a whole pile of applications, but before that - some fun! Let's look at the general properties of 'modern' drugs, and see what general patterns emerge - for example are drugs orally dosed, what fraction are antibodies, etc. Of course, these analyses are clearly limited by small numbers, but I think they provide useful guideposts for the changing landscape of therapeutic discovery (we have a more extensive analysis of these sort of things underway) and would be happy to share ms drafts with interested parties.
So, I have a paper review to finish off, and then assess a whole pile of applications, but before that - some fun! Let's look at the general properties of 'modern' drugs, and see what general patterns emerge - for example are drugs orally dosed, what fraction are antibodies, etc. Of course, these analyses are clearly limited by small numbers, but I think they provide useful guideposts for the changing landscape of therapeutic discovery (we have a more extensive analysis of these sort of things underway) and would be happy to share ms drafts with interested parties.
The dataset I have looked at are drugs (specifically here NMEs, so novel chemical structures approved for the first time) launched in the US since Jan 1st 2007 and up until July 31st 2009 (so the past 31 months) there are 58 drugs in this set (mail me if you want some spreadsheets, etc) so not a huge number, but that's what there is. This is an average of 1.9 new drugs approved per month. Of these 58, six are not therapeutic drugs (but are imaging agents, probes, etc.), these six non-therapeutics were removed from further analysis, and all further counts refer to a therapeutic subset of 52 drugs.
Drug Class
Ten of the 52 drugs (19.2%) are proteins/peptides. Of these four are monoclonal antibodies, one is an enzyme, two are peptides, and three are recombinant proteins. The peptides can be readily accessed synthetically, and so only eight drugs (15.4%) can be considered to be biotechnology products. This leaves 42 drugs that are small molecule therapeutics.
Natural Products (and derived from Natural Products)
Of the 42 'small molecule' drugs, eight are 'natural products', defined here as either being, or very similar to, natural primary or secondary metabolites. If one considers that the peptide drugs (since they are directly related to naturally occurring molecules) are natural products as well, then 34.6% of drugs are 'Natural Product derived', however, for a more sensible definition (excluding peptides and proteins, 15.4% of drugs are 'natural products').
Dosing Route
Of the 52 therapeutic drugs, five (9.6%) are topically dosed, 18 (34.6%) are parenterally dosed, and 29 (55.8%) are orally dosed. When peptide/protein drugs are excluded from these counts, five are topically dosed, eight are parenterally dosed and 29 are orally dosed.
Black Boxes
Of the 52 therapeutic drugs, 21 (40%) had a black box warning on launch. These are equally distributed between biological (four out of ten) and small molecule (16 out of 42).
Rule-Of-Five
Of the 42 therapeutic small molecule drugs, 28 (66.7%) pass the Rule-of-Five and 14 (33.3%) fail the Rule-of-Five. It is of interest to examine the relationship between Rule-of-Five pass/fail, and oral/non-oral delivery - of the 29 Orally dosed drugs, 20 (69%) pass the rule-of-five. The data is summarised in a table below.
Orally Dosed Non Orally-Dosed total Pass Rule-of-Five 20 8 28 Fail Rule-of-Five 9 5 14 total 29 13 42 Targets
Five of the therapeutic drugs were first-in-target (9.6%), while an additional 41 have a previously 'drugged' target/established mechanism of action (MoA) and finally six (11.5%) have either an unknown, or non-targeted MoA. Of the 'newly-drugged targets for this period, four out of five have 'serum exposed' binding sites (i.e. they are secreted proteins or membrane-bound receptors), and one target is nuclear localised (but is not a human genome target).
-
New Drug Approvals - Pt. XIII - Saxagliptin (Onglyza)

 On the 31st July 2009 Saxagliptin (tradename Onglyza) was approved for the treatment of Type II diabetes - Type 2 Diabetes is also known as adult-onset diabetes, and also non-insulin-dependent diabetes melittus (NIDDM). It is the type of diabetes that is often associated with obesity, and so is an increasingly common disease/condition in our well-fed western and also developing world cultures.
On the 31st July 2009 Saxagliptin (tradename Onglyza) was approved for the treatment of Type II diabetes - Type 2 Diabetes is also known as adult-onset diabetes, and also non-insulin-dependent diabetes melittus (NIDDM). It is the type of diabetes that is often associated with obesity, and so is an increasingly common disease/condition in our well-fed western and also developing world cultures.
Saxagliptin (previously known by the research code BMS-477118) is the third orally-dosed Dipeptidyl peptidase-IV (or DPP-IV) inhibitor to market, and is in the same mechanistic class as other 'gliptins' - Sitagliptin (tradename Januvia) and Vildagliptin (tradename Galvus/Eucreas) which are both launched and also others such as Alogliptin (aka SYR322) and Linagliptin (aka BI-1356, and expected tradename Ondero), which are in late stage clinical trials. The DPP-IV drug class has had quite a complex development and commercial history, as web searches will readily show.
Saxagliptin is a small molecule drug (Molecular Weight of 315.4 g.mol-1 for Saxagliptin itself, and 334.43 g.mol-1 for the Saxagliptin monohydrate dosed ingredient), and has low aqueous solubility. Saxagliptin is well absorbed and has low plasma protein binding (<30%),>ca. 15.8 µmol) once a day. The full prescribing information can be found here.
The Saxagliptin structure is (1S,3S,5S)-2-[(2S)-2-Amino-2-(3-hydroxytricyclo[3.3.1.13,7]dec-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile. It contains a number of interesting chemical groups, and a clear underlying similarity to a dipeptide can be seen in the 2-D structure (the enzyme DPP-IV, a proteinase, cleaves the two N-terminal amino acids of its substrate peptides)). Normally DPP-IV is involved in the inactivation of two endogenous peptides, GLP-1 and GIP, by DPP-4, blocking this degradation potentiates the secretion of insulin in the beta cells and suppress glucagon release by the alpha cells of the islets of Langerhans located in the pancreas. The first functional group of note is the nitrile (the triple bonded nitrogen-carbon unit) - this is essential to the inhibitory activity and is found in several of the other 'gliptins. This group forms a reversible, covalent bond with the residue Ser 630 of DPP-IV. Secondly, there is the bulky, hydrophobic adamantane (or (tricyclo[3.3.1.13,7]decane) group (this is the 3-D cage like portion of the molecule. Simple substituted adamantanes are sometimes drugs in their own right, for example amantadine, memantine and rimantadine. Within the 'gliptins though, the large bulky adamantyl group blocks an intramolecular cyclisation, which inactivates the inhibitor. These nitrile and adamantyl groups are linked via an amide bond, and an unusual 5,3 fused ring system pyrollidine (which resembles the amino-acid proline, found in the corresponding position of natural substrates).
Saxagliptin canonical SMILES: C1CC2(CC3CC1C(C3)(C2)O)C(C(=O)N4C(CC5C4C5)C#N)N Saxagliptin InChI: InChI=1S/C18H25N3O2/c19-8-13-4-11-5-14(11)21(13)16(22)15(20)17-2-1-12-3-10(6-17)7-18(12,23)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12?,13-,14-,15+,17?,18?/m0/s1 Saxagliptin InChIKey: SBBHGAZNWZOMBJ-TXTOARCRSA-N Saxagliptin CAS registry: 361442-04-8 Saxagliptin ChemDraw: Saxagliptin.cdx
The license holder for Saxagliptin is Bristol Myers Squibb and the product website is www.onglyza.com.
-
Small Molecule Bioactivity Course At The EMBL-EBI, 25th Jan 2010
 The registration for the small molecule bioactivity resources course to be held at the EMBL-EBI from Monday 25th January to Friday 29th January 2010, is now open. We will have some special guest speakers, and the aim is to make it very interactive with a focus on problem solving, so it should be good. There are a number of bursaries available so it could even be free!
The registration for the small molecule bioactivity resources course to be held at the EMBL-EBI from Monday 25th January to Friday 29th January 2010, is now open. We will have some special guest speakers, and the aim is to make it very interactive with a focus on problem solving, so it should be good. There are a number of bursaries available so it could even be free! -
EMBL-EBI Open Day - 3rd November 2009
 There is an Open Day at the EMBL-EBI on Tuesday the 3rd November, our group will have a poster, and we may even bake some of our special cakes - a little bit like a Karelian pie (Karjalan Piirakka), but with 'angel-hair' turnip instead of a potato filling, if we have some time. Despite this offer, please check out the web link and register if you are interested in attending.
There is an Open Day at the EMBL-EBI on Tuesday the 3rd November, our group will have a poster, and we may even bake some of our special cakes - a little bit like a Karelian pie (Karjalan Piirakka), but with 'angel-hair' turnip instead of a potato filling, if we have some time. Despite this offer, please check out the web link and register if you are interested in attending. -
Hit-the-sack - Pt XI. Tilton School, Tilton, New Hampshire
This was the accommodation for the 2009 Gordon Research Conference on Computer-Aided Drug Design, it was my first States-based Gordon Conference. I guess you can't book in to stay here (unless you want an education, and are under 18 or so) other than staying there as part of a Gordon Conference....
The conference was very, very good, but as protocol demands I can't tell you about that, but I can tell you about the room, the stay and the extra-curricular activities.
The school website is here
In summary a score of 68% - russulatastic!
- Room Quality - 1/10 Spartan is the word, twin single beds, nylon carpet, desks and cupboards with the ability to padlock them (bring a padlock if you want to make things secure). Some rooms had fans (mine didn't) so expect a hot night when it is warm. Communal bathrooms, with 'youth sized' shower cubicles. Europeans who will probably wake early, had better turn the taps on about 15 minutes before they want to wash/shower with warm water. Here is some additional advice (pack pyjamas (or the equivalent), pack twice as many clothes as you think you will need, pack shampoo, conditioner, soap, and maybe even a towel and robe. Pack insect repellant (DEET is the only stuff that works, apparently, also take anti-itch cream). Prepare yourself mentally to potentially share a room with an adult you do not know.
- Getting There - 5/10 A couple of hours by bus from Boston, maybe an hour from Manchester NH airport.
- Cost - 8/10 - paid as part of the conference fees - very good value for money in my view.
- Phone reception - 2/10 - Very patchy signal, no pay-phones nearby.
- Internet - 8/10 - Good fast internet, both wireless (in my room, but not all) and ethernet. However, the IP range of the network there was the same as my works, so I had some odd behaviors....
- Conference facilities - 8/10. Good, high quality facilities, projectors, wireless networking in the conference room, good acoustics, well equipped (there is however a striking picture on the wall of one of the rest-rooms in the conference hall).
- Mushroom factor - 9/10 - In a word - Fantastic - many new species for me, loads of russulas, amanitas, boletes, and excellent amphibian fauna too. If you go into the woods at all - take insect repellant for the mosquitos - West Nile virus and Eastern Equine Encephalitis are very, very minor risks there, but most of all they love blood and they are very strong and hungry lil fellas. If you want to take photos at ground level (where the majority of the mozzies seem to loiter), expose no patches of skin, and even consider wearing cotton gloves (seriously). There are also a lot of ticks, so long trousers and socks are an essential. Unlike the UK, there is no 'right to roam', so make sure you have permission to forage.












