-
Course: Small Molecule Bioactivity Resources at the EMBL-EBI
 Sorry for not having posted for so long, but some good news. There will be a residential course held at the EMBL-EBI from Monday 25th January to Friday 29th January 2010, the agenda will focus on applications of SAR databases to biological problems, but of course we'll also include contributions from many other faculty at the EMBL-EBI to illustrate how 'it all fits togther'. We hope to have eight full bursaries available for the course, and registration will open at the end of this July. I'll post further details on the chembl-og when there is more available.....
Sorry for not having posted for so long, but some good news. There will be a residential course held at the EMBL-EBI from Monday 25th January to Friday 29th January 2010, the agenda will focus on applications of SAR databases to biological problems, but of course we'll also include contributions from many other faculty at the EMBL-EBI to illustrate how 'it all fits togther'. We hope to have eight full bursaries available for the course, and registration will open at the end of this July. I'll post further details on the chembl-og when there is more available..... -
New Drug Approvals - Pt. IX - Besifloxacin (Besivance)
Also on the market this year is Besifloxacin Hydrochloride (USAN) which is marketed as Besivance, approved on May 28th. Besifloxacin is a fluoroquinolone antimicrobial indicated for the treatment of bacterial conjunctivitis, and competes against other fluoroquinolones for the ophthalmic treatment of bacterial infections. Besifloxacin has activity against Gram-positive and Gram-negative bacteria and acts through inhibition of both bacterial DNA gyrase and topoisomerase IV. Besifloxacin is known to be active against various Corynebacterium, Haemophilus, Moraxella, Staphylococcus and Streptococcus spp. Besifloxacin is a small molecule drug (Molecular Weight of 393.8 g.mol-1 for Besifloxacin itself, and 430.3 g.mol-1 for the HCl salt), is Rule-of-Five compliant and is delivered as an ophthalmic suspension. Besifloxacin has a plasma half-life is of 7 hours. Recommended dosage is one drop instilled in the affected eye(s) 3 times a day, four to twelve hours apart, for 7 days. The full prescribing information is here.
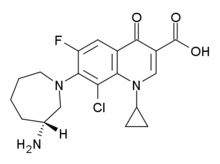
The structure (+)-7[(3R)-3-aminohexahydro-1H-azepin-1-yl]-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid) contains both an amine and a carboxylic acid functional group, and so will normally exist as a zwitterion. Overall, Besifloxacin is a rigid molecule with few rotatable bonds. The fluoroquinolone class of antibiotics is quite extensive, and is named after the fluro-substituted, fused six-six ring structure at the center of the molecule containing a nitrogen and also the aryl-ketone (the 'one' part of the name).
Besifloxacin Canonical SMILES: N[C@@H]4CCCCN(c3c(F)cc2c(=O)c(C(=O)O)cn(C1CC1)c2c3Cl)C4 Besifloxacin InChI: InChI=1S/C19H21ClFN3O3/c20-15-16-12(18(25)13(19(26)27)9-24(16)11-4-5-11)7-14(21)17(15)23-6-2-1-3-10(22)8-23/h7,9-11H,1-6,8,22H2,(H,26,27) Besifloxacin InChIKey: QFFGVLORLPOAEC-UHFFFAOYSA-N Besifloxacin CAS registry: 141388-76-3 Besifloxacin ChemDraw: Besifloxacin.cdx
The manufacturer of Besifloxacin is Bausch & Lomb, Inc. and the product website is www.besivance.com.
-
New Drug Approvals - Pt. VIII - Iloperidone (Fanapt)
 Another drug onto the market this year is Iloperidone (USAN) marketed as Fanapt, which was approved on May 6th. Iloperidone is an antipsychotic agent indicated for the acute treatment of schizophrenia in adults. Iloperidone displays broad polypharmacology, acting as an antagonist at dopamine D2 (Ki of 6.3 nM) and dopamine D3 (Ki of 7.1 nM), serotonin 5-HT2A (Ki of 5.6 nM), dopamine D4 (Ki 25 nM), 5HT6 (Ki of 43 nM), 5HT7 (Ki of 22 nM) and alpha-1 adrenergic receptor (Ki of 36nM) (phew!) and belongs to the general class of atypical antipsychotics. Lower affinities, but also probably relavent for the clinical pharmacology, are observed at the 5HT1A, D1 and H1 receptors). Iloperidone is a small molecule drug (Molecular Weight of 426.5 g.mol-1), is fully Rule-of-Five compliant, lipophilic and pratically insoluble in water and has good oral absorption (96% bioavailable). Iloperidone has a plasma half-life of 18 hours, a volume of distribution of 1,340-2,800L and plasma protein binding of ~95%.
Another drug onto the market this year is Iloperidone (USAN) marketed as Fanapt, which was approved on May 6th. Iloperidone is an antipsychotic agent indicated for the acute treatment of schizophrenia in adults. Iloperidone displays broad polypharmacology, acting as an antagonist at dopamine D2 (Ki of 6.3 nM) and dopamine D3 (Ki of 7.1 nM), serotonin 5-HT2A (Ki of 5.6 nM), dopamine D4 (Ki 25 nM), 5HT6 (Ki of 43 nM), 5HT7 (Ki of 22 nM) and alpha-1 adrenergic receptor (Ki of 36nM) (phew!) and belongs to the general class of atypical antipsychotics. Lower affinities, but also probably relavent for the clinical pharmacology, are observed at the 5HT1A, D1 and H1 receptors). Iloperidone is a small molecule drug (Molecular Weight of 426.5 g.mol-1), is fully Rule-of-Five compliant, lipophilic and pratically insoluble in water and has good oral absorption (96% bioavailable). Iloperidone has a plasma half-life of 18 hours, a volume of distribution of 1,340-2,800L and plasma protein binding of ~95%.
Iloperidone elimination is mainly through CYP dependent hepatic metabolism (specifically CYP3A4 and CYP2D6); some of these metabolites are themselves pharmacologically active. CYP2D6 is an example of a genetically variable gene, with a fraction of the population having a form of the gene that is significantly less active - so called 'slow metabolizers', this generally leads to variability of drug response. Iloperidone has an apparent clearance of 47-102 L/hour, with the bulk of the clearance being renal (i.e.. the drug is excreted in the urine). The recommended dosage is 12 to 24 mg/day administrated twice daily (equivalent to ca. 28-56uM). Like many drugs of this class, there are a broad range of potential adverse events, including a propensity for the compound to increase QTc interval. The full prescribing information can be found here.
Iloperidone has a boxed warning (colloquially known as a 'black box').
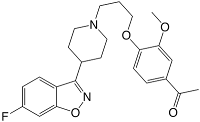
Iloperidone belongs to the chemical class of piperidinyl-benzisoxazole derivatives - the piperidine in the six membered ring containing the nitrogen in the middle of the molecule, while the benzisoxazole is the fused five-six dual ring structure at the bottom left. Its structure 4'-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidino]propoxy]-3'-methoxyacetophenone contains a tertiary amine which makes the molecule basic, but otherwise the molecule is largely lipophilic in character. A relatively unusual chemical feature for a drug is the presence of the aryl-ketone group.
Iloperidone canonical SMILES: O=C(c4ccc(OCCCN3CCC(c2noc1cc(F)ccc12)CC3)c(OC)c4)C Iloperidone InChI: InChI=1/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-2 7-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17 H,3,8-13H2,1-2H3 Iloperidone InChIKey: XMXHEBAFVSFQEX-UHFFFAOYAT Iloperidone CAS registry: 133454-47-4 Iloperidone ChemDraw: Iloperidone.cdx
The license holder for Iloperidone is Vanda Pharmaceuticals and the product website is www.fanapt.com
-
Recession Busting For the Careful Academic: Pt 1. UK Rail Fares
The way that train journey tickets are priced in the United Kingdom is non-rational. There appears to be relatively little correlation between journey length and price paid for a ticket, and it is quite galling to sit on a train and happen to catch sight of someone’s equivalent ticket for yours and see they have paid a lot less for the same seat on the same train. When I go into McDonald's for a petit salad, or Starbuck's for an Orange Mocha Frappuccino, I am secure in knowing that other customers in the same store will pay the same as me for the same product and service - all is right with the world; when it comes to trains (and planes) as a consumer, I am always left with the impression that I haven't necessarily got the best deal. The basic reason for this (for trains) is that for some time now, train tickets have been ‘market priced’ (sic), which effectively means that the companies can charge what they like for a particular journey, albeit that there is some statutory regulation on price increases.
I travel to work by train every day, and my ticket is (or used to be) 16.00 GBP per day. I think this is quite a lot of money (equivalent to a whole weeks worth of lunches at the excellent campus canteen, or two typical contemporary Compact Discs by popular recording artists, etc.).
Anyway, here is the money saving tip - try ‘ticket splitting’. Using this simple approach I saved 1.80 GBP per day (so an incredible 12% saving), for the same seat on the same train at the same time. The basic idea is that you investigate the prices for a ticket that splits your journey at an intermediate station. You then buy these two tickets in place of one. For ca. 80% of the split ticket journeys for my normal train there was a cost saving, (for 20% the journey was more expensive). A general heuristic would seem to be to look for splits either side of ‘popular’ stations - but even a brute force approach is not that difficult for typical journeys.
It is actually quite difficult to find out from official sources and customer agents what the lowest price ticket(s) for your journey are, as expected they are all motivated to encourage you to pay the highest price you are prepared to pay. I was reliably informed it would ‘take a super-computer’ to work out the best fare (really?, especially when some physical possibility heuristics are applied). Being the sort of person I am, I phoned the regulators, train operators and so forth, and basically it is down to you to do the investigative work. So when you see the words ‘cheapest fare’ on a website, there may be something cheaper still!
There are some important restrictions (primarily that the train you are actually travelling on must stop at the ‘split’ station). Please check your terms and conditions of travel, etc., etc., ....
-
New Drug Approvals - 2008 Retrospective
I put together a graph this afternoon, from home, with no real network connection, no database access and a sick dog - so treat the rendering of the data as preliminary, but I think it is quite interesting. Anyway, below is a retrospective view of 2008 US drug approvals. 10 Orals, 13 Parenterals and 1 Topical. 2 Natural Product derived drugs (excluding peptide drugs). All is well with the Rule of Five. 6 with black-box warnings. 1 new target (but quite a few with unknown mode of action). 20 small molecules, and 4 peptide/protein drugs.....
-
New Drug Approvals - Pt. VII - Canakinumab (Ilaris)
 Next in our series of posts on new FDA drug approvals this year is Canakinumab, approved on the 18th of June. Canakinumab is a Interleukin-1 beta (IL-1β) blocker indicated for the treatment of various auto-immune diseases - specifically described by the term Cryopyrin-Associated Periodic Syndromes (CAPS), including Familial Cold Autoinflammatory Syndrome (FCAS) (alternatively known as familial cold urticaria) and Muckle-Wells Syndrome (MWS). These are rare (albeit debilitating) genetic diseases, and are usually caused by mutations in the NLRP-3 gene - here is the link to the OMIM entry for NLRP-3.
Next in our series of posts on new FDA drug approvals this year is Canakinumab, approved on the 18th of June. Canakinumab is a Interleukin-1 beta (IL-1β) blocker indicated for the treatment of various auto-immune diseases - specifically described by the term Cryopyrin-Associated Periodic Syndromes (CAPS), including Familial Cold Autoinflammatory Syndrome (FCAS) (alternatively known as familial cold urticaria) and Muckle-Wells Syndrome (MWS). These are rare (albeit debilitating) genetic diseases, and are usually caused by mutations in the NLRP-3 gene - here is the link to the OMIM entry for NLRP-3.
Canakinumab is the third Il-1β blocker to reach the market, and as the INN/USAN suggests it is a human (the 'u' before mab) derived monoclonal antibody (the 'mab' part). The earlier IL-1β blockers were Anakinra (Kineret), and Rilonacept (Arcalyst); both of these earlier therapies are not antibody therapies, and have different detailed mechanisms of action. Canakinumab binds directly to IL-1β, and is reported to not bind to the related IL-1α and IL-1ra proteins. Rilonacept similarly binds directly to IL-1β, but also binds to the related IL-1α and IL-1ra proteins with modest selectivity (with binding constants ~3 and ~12 fold down relative to IL-1β respectively).
Canakinumab (previously known as the research code ACZ885) is a human monoclonal antibody that exhibits multiple glycoforms with a deglycosylated molecular weight of ca. 145 kDa. Canakinumab has a good subcutaneous (s.c.) absorption (ca. 70% bioavailable), a plasma half-life of ca. 4 weeks, a volume of distribution of ca. 86 mL/kg and a systemic clearance of 2.5 mL/day/kg. The recommended dosage of 150 mg is administrated by subcutaneous injection just once every two months. The full prescribing information can be found here.
The CAPS set of diseases are rare (about 1 in a million in the US), and Ilaris will unlikely have significant commercial impact, despite the high prices that are often charged for this type of 'orphan' drug (for example, Cerezyme is about $200,000 per patient p.a.). However, the commercial strategy of getting initial approval for a orphan disease is quite interesting and common. Basically it is to target a tightly defined genetic disease, get a drug that works against that mechanism (as detailed above, it does not have to be the gene that is mutated in the disease itself, just some member of the signalling pathway/mechanism that involves that gene), get approval, and then study new potential indications (the key target, IL-1β, has been implicated, and clinically shown, to be important in many, many human diseases - for example, type-2 diabetes (which is a big and growing disease), gout, and juvenile idiopathic arthritis). If the studies show utility in these more common diseases, there is great potential to 1) develop genuinely innovative medicines against more frequent diseases and 2) grow revenues. There are some issues with this strategy though.... but that is for another day.
Canakinumab molecular formula: C6452H9958N1722O2010S42
Canakinumab CAS registry: 914613-48-2
The license holder for Canakinumab is Novartis. and the product website is www.ilaris.com.
-
New Drug Approvals - Icons
 To make things a little simpler, and to allow rapid visual comparisons, we have set up a series of icons for our New Drug Approvals posts. These are very simple, and should be self explanatory.
To make things a little simpler, and to allow rapid visual comparisons, we have set up a series of icons for our New Drug Approvals posts. These are very simple, and should be self explanatory.
Three examples should make everything clear.
A small molecule, Rule-of-Five compliant, oral drug
A large molecule, parenteral drug, against a new target, with a black box warning.
A natural product-derived topical drug
Well maybe things are not as clear as could be..... but hey, you guys and gals are smart, and will soon work it out; and I am sure the magic blog pixies will add the correct icons to the previous posts.
-
New Team Member :)