-
New Drug Approvals - Pt. III - Golimumab (Simponi)

 Third on our series of posts on new FDA drug approvals this year is Golimumab, approved on the 24th of April. Golimumab is a tumor necrosis factor alpha (TNFα) blocker indicated for the treatment of active forms of rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Golimumab is the fourth TNF inhibitor, after Etanercept, Infliximab and Adalimumab, to reach the market. Etanercept is a fusion protein of an engineered from the the TNF receptor fused to IgG1, while Infliximab and Adalimumab are very similar to Golimumab structurally in that they are all 'conventional' monoclonal antibodies. Golimumab (previously known as CNTO-148) is a human monoclonal antibody that exhibits multiple glycoforms with molecular weights of ca. 151 kDa. Golimumab has a good subcutaneous (sc) absorption (ca. 53% bioavailable), a plasma half-life of ca. 2 weeks, a volume of distribution of 58 to 126 mL/kg and a systemic clearance of 4.9 to 6.7 mL/day/kg. The recommended dosage of 50 mg is administrated by subcutaneous injection just once a month. The full prescribing information can be found here.
Third on our series of posts on new FDA drug approvals this year is Golimumab, approved on the 24th of April. Golimumab is a tumor necrosis factor alpha (TNFα) blocker indicated for the treatment of active forms of rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Golimumab is the fourth TNF inhibitor, after Etanercept, Infliximab and Adalimumab, to reach the market. Etanercept is a fusion protein of an engineered from the the TNF receptor fused to IgG1, while Infliximab and Adalimumab are very similar to Golimumab structurally in that they are all 'conventional' monoclonal antibodies. Golimumab (previously known as CNTO-148) is a human monoclonal antibody that exhibits multiple glycoforms with molecular weights of ca. 151 kDa. Golimumab has a good subcutaneous (sc) absorption (ca. 53% bioavailable), a plasma half-life of ca. 2 weeks, a volume of distribution of 58 to 126 mL/kg and a systemic clearance of 4.9 to 6.7 mL/day/kg. The recommended dosage of 50 mg is administrated by subcutaneous injection just once a month. The full prescribing information can be found here.
Golimumab has a boxed warning (colloquially known as a 'black box').
Golimumab is a monomeric immunoglobulin (IgG) consisting of four polypeptide chains in a "Y"- shaped form: two identical heavy chains of ~450 aminoacids and two identical light chains of ~217 animoacids, connected by disulfide bonds.
Golimumab molecular formula: C6530H10068N1752O2026S44
Golimumab CAS registry: 476181-74-5
The license holder for Golimumab is Centocor Ortho Biotech Inc. and the product website is www.simponi.com.
-
More On Biological Drugs.....
So, I cleaned up a few stragglers in terms of the classification of therapeutic class, and the data for the 551 discussed below is....
class count peptides and proteins 521 oligonucleotides 17 oligosaccharides 13 So this has pushed the fraction of protein/peptide biological INNs up to 95%. Here is a pretty graph.
Of course, the thing I should have done first of all, is to look at currently approved agents under the same classification scheme......
-
Biological Drugs?
 I was on the M4 recently, and we saw an M3 with a cool geeky numberplate, as we were discussing blockade of M2 for swine flu, if only there was an M1 to complete the pattern. Anyway, being the sort of father keen to impress their kids with my exciting life, I took a picture - the kids weren't impressed.
I was on the M4 recently, and we saw an M3 with a cool geeky numberplate, as we were discussing blockade of M2 for swine flu, if only there was an M1 to complete the pattern. Anyway, being the sort of father keen to impress their kids with my exciting life, I took a picture - the kids weren't impressed.
The world is full of hope for the bright future for biological therapies - monoclonal antibodies, solubilised receptors, gene therapy, etc. I've been trying to estimate the size of the biological drug portion of CandiStore, and so here is some data on late stage clinical candidates (i.e. those that had been granted INNs) skimmed from a WHO review document from late 2007. The general pattern will not have changed significantly for 2008 and early 2009.
There are 551 total 'biological' INNs, of which 519 are protein (or glycoprotein) - so 94% of biological drugs are proteins. The breakdown into functional classes for these protein drugs is as follows....
So monoclonal antibodies are the largest single class (at 29%) - not quite as high as I had expected; other interesting general features are the 12% of biological INNs that are enzymes, so these will address 'loss of function' deficiencies, which are impossible to achieve directly with a small molecule agent. Furthermore, seven distinct functional classes of INNs make up three quarters of the total. Finally, the number of launched monoclonal antibody drugs is 34 (not including the most recent Golimumab (or the TradeName Simponi), however, not all of these are therapeutic antibodies (some are imaging agents), so just under a quarter of monoclonal antibody drugs that have been granted an INN have been approved (so far, in the U.S.A, etc., etc.)
More on these trends another day.....
-
New Drug Approvals - Pt. II - Febuxostat (Uloric/Adenuric)

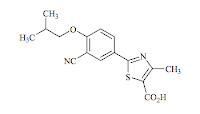
 Second onto market this year is Febuxostat, approved on the 13th of February. Febuxostat is a xanthine oxidase inhibitor used for the treatment of hyperuricemia (gout), and competes against well established agents such as Allopurinol (Allopurinol was approved in 1964 for the treatment of gout). Febuxostat is a small molecule drug (Molecular Weight of 316.4 g.mol-1) is fully Rule-Of-Five compliant, is essentially insoluble in water, and has good oral absorption (>49% bioavailable). Febuxostat plasma half-life of 5 to 8 hours, a volume of distribution of 50L, high plasma protein binding at 99.2% (unsurprising given the lipophilic acid nature of the molecule). Febuxostat is extensively metabolised, both by the UGT system, producing glucoronidated metabolites, and also by a range of complex CYP mediated transformations (metabolites of metabolites....). Some of the CYP mediated metabolites involve hydroxylation of the butyl sidechain, these themselves are pharmacologically active against the target. Roughly half the dose is cleared renally, and the other half in the faeces. Typical daily dosing is 40mg (or ~126µmol), or 80mg if insufficient efficacy is observed. The full prescribing information is here.
Second onto market this year is Febuxostat, approved on the 13th of February. Febuxostat is a xanthine oxidase inhibitor used for the treatment of hyperuricemia (gout), and competes against well established agents such as Allopurinol (Allopurinol was approved in 1964 for the treatment of gout). Febuxostat is a small molecule drug (Molecular Weight of 316.4 g.mol-1) is fully Rule-Of-Five compliant, is essentially insoluble in water, and has good oral absorption (>49% bioavailable). Febuxostat plasma half-life of 5 to 8 hours, a volume of distribution of 50L, high plasma protein binding at 99.2% (unsurprising given the lipophilic acid nature of the molecule). Febuxostat is extensively metabolised, both by the UGT system, producing glucoronidated metabolites, and also by a range of complex CYP mediated transformations (metabolites of metabolites....). Some of the CYP mediated metabolites involve hydroxylation of the butyl sidechain, these themselves are pharmacologically active against the target. Roughly half the dose is cleared renally, and the other half in the faeces. Typical daily dosing is 40mg (or ~126µmol), or 80mg if insufficient efficacy is observed. The full prescribing information is here.
The structure (2-[3-cyano- 4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid) contains a nitrile group, and a free carboxylic acid group (which will make the drug negatively charged under physiologic conditions and dominate its physical chemistry). The molecule is quite rigid and comparatively planar (due to the presence of sp2 hybridized atoms adjacent to the core phenyl and thiazole rings). A nitrile is quite unusual in drugs due to its comparative susceptibility to nucleophilic attack, and also metabolic stability - nitriles can be hydrolysed to carboxylic acids.
Febuxostat canonical SMILES: CC1=C(SC(=N1)C2=CC(=C(C=C2)OCC(C)C)C#N)C(=O)O Febuxostat InChI: InChI=1S/C16H16N2O3S/c1-9(2)8-21-13-5-4-11(6-12(13)7-17)15-18-10(3)14(22-15)16(19)20/h4-6,9H,8H2,1-3H3,(H,19,20) Febuxostat InChIKey: BQSJTQLCZDPROO-UHFFFAOYSA-N Febuxostat CAS registry: 144060-53-7 Febuxostat Chemdraw: Febuxostat.cdx
The manufacturer of Febuxostat is Takeda Pharmaceuticals and the product website is www.uloric.com
-
New Drug Approvals - Pt. I - Milnacipran (Ixel/Savella)
An occasional series of posts with news and top level details on new FDA drug approvals. The series is a little retrospective at the moment, but it will not take much time to catch up for 2009.
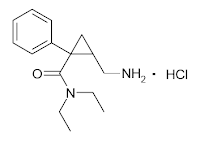
First 'out of the gates' on 14th Jan this year is an 'old' compound, Milnacipran (Savella in the US) for the treatment of fibromyalgia syndrome. Milnacipran is an SNRI (Serotonin Norepinephrine Reuptake Inhibitor, and as such blocks the function of the norepinephrine transporter NET and the serotonin transporter SERT), like all compounds of this pharmacological class, the molecule is small (Molecular Weight of 246.4 g.mol-1 for Milnacipran itself, and 282.2 g.mol-1 for the HCl salt) is fully Rule-Of-Five compliant, has high aqueous solubility, and also has good oral absorption (85% bioavailable) and metabolism characteristics. (A plasma half-life of ca. 8 hours, a volume of distribution of 400L, low plasma protein binding at 13%. Excretion is renal (through the kidneys and urine) and excretion is primarily of the unchanged drug (55% unchanged). With a molecule like this, it should come as no surprise that it is delivered orally. Recommended dosage is 100mg (or ~354µmol, or ~177µmol of the pharmacologically active enantiomer, see below) per day, although dosing is phased from a low dose to 'full' dose over a week. Milnacipran is more potent at blocking norepinephrine uptake than serotonin uptake. The full prescribing information is here.
Milnacipran has a boxed warning (colloquially known as a 'black box').
Notable features of the chemical structure ((±)-[1R(S),2S(R)]-2-(aminomethyl)-N,N-diethyl-1- phenylcyclopropanecarboxamide hydrochloride) are the primary amine (the NH2) which is basic (it can be protonated and therefore positively charged), which will dominate its physicochemical properties. The remainder of the molecule is quite lipophillic. Of further note is the cyclopropane ring (the triangle part in the middle), which is completely rigid and will dominate the three-dimensional properties (shape) of the drug. The functional groups attached to the cyclopropane are attached with mixed stereochemistry, so the drug is 'racemic' - for this reason stereochemistry is not explicitly shown on the the 2-D structure below. However, the stereoisomers have different pharmacological activity, with the 'active' d-isomer having different properties (e.g. a longer half-life) to the 'inactive' l-isomer Finally, the approved drug is not Milnacipran itself, but the Hydrochloride salt (so in the tablet the amine is indeed protonated, as discussed above).
Milnacipran canonical SMILES: CCN(CC)C(=O)C1(CC1CN)C2=CC=CC=C2 Milnacipran InChI: InChI=1S/C15H22N2O/c1-3-17(4-2)14(18)15(10-13(15)11-16)12-8-6-5-7-9-12/h5-9,13H,3-4,10-11,16H2,1-2H3 Milnacipran InChIKey: GJJFMKBJSRMPLA-UHFFFAOYSA-N Milnacipran HCl CAS registry: 101152-94-7 Milnacipran CAS registry: 92623-85-3 Milnacipran (two enantiomers) Chemdraw: Milnacipran.cdx
I mentioned it was an old compound, well it is, just that old is relative. The SNRI/SSRI therapeutic classes are old ones, and several drugs of these related classes are now available generically. SSRIs and SNRIs are primarily 'antidepressant' drugs, and this compound itself has been used in certain countries for some time (Austria, since 1998, and also at least Chile and Israel), there has been much interest in expanding the clinical use of SSRIs/SNRIs beyond their original antidepressant actions. As one would expect, the commercial history of Milnacipran appears quite complicated, and the latest approval is for a 'new' therapeutic indication - fibromyalgia (chronic and widespread muscle pain). Fibromyalgia is a complicated disease with several competing theories for its causes, and is often associated/comorbid with depression.
The license holder for Milnacipran is Cypress Bioscience and the product website is www.savella.com
Finally, given the age of the compound, it would be interesting to know what the patent status of the compound is?
-
Software: OmniGraphSketcher
 Here is a software tool that I can't believe didn't exist before - maybe it did, and I didn't know, but it is good - as good as an apple pie, that tastes very nice.
Here is a software tool that I can't believe didn't exist before - maybe it did, and I didn't know, but it is good - as good as an apple pie, that tastes very nice.
Many times there is the need to produce illustrative graphs, annotated graphs of imported data, etc. This is a tool that almost does everything I need, Having the ability to do pie-charts would make this perfect, maybe it does them and I haven't found the options yet. But compared to how I struggle with Excel's graphing options sometimes, this is a different world.
There are some videos of OmniGraphSketcher on the OmniGroup website, that show its capabilities pretty well. The software is licensed, but comes with a generous 30 day free trial, and the price is $29.95 for a full license. Student and faculty (Yey!!) discounts are available, as is a family pack and volume discounts. So students with large families, will get an incredibly keen price.
-
Hit-the-sake - Pt. X - Marriott Courtyard, Neuilly-sur-seine
 I was here for a weekend meeting in Paris. The meeting was great, some really, really interesting people, and a nice crisp pace to discussions, agreements and actions. I hope I picked up a few hints! The hotel is in a suburb of Paris, but close enough to walk the L'arc de Triomphe, Champs Elysees, etc. The other great thing about the trip was just how good the Eurostar is now, especially that the St. Pancras terminal is fully functional. The only thing to watch out for are all the 'helpers' at Gare de Nord when you arrive - I didn't, but I will next time! Oh, and now the pound and Euro have parity, it is so, so expensive on the continent.
I was here for a weekend meeting in Paris. The meeting was great, some really, really interesting people, and a nice crisp pace to discussions, agreements and actions. I hope I picked up a few hints! The hotel is in a suburb of Paris, but close enough to walk the L'arc de Triomphe, Champs Elysees, etc. The other great thing about the trip was just how good the Eurostar is now, especially that the St. Pancras terminal is fully functional. The only thing to watch out for are all the 'helpers' at Gare de Nord when you arrive - I didn't, but I will next time! Oh, and now the pound and Euro have parity, it is so, so expensive on the continent.
An overall score of 44%, let down by cost and internet.
- Room Quality - 3/10 OK Room, but despite the fact that I had a non-smoking room, someone had been smoking in there.
- Getting There - 5/10 About a £15 cab ride from Gare de Nord, nice quick roads and takes about 20 minutes.
- Cost - 3/10 - ca. 450 Euro rate on the inside of the door. I cried. Hopefully the meeting got a good rate on the room. The breakfast and food were a delight though.
- Phone reception - 6/10 - Good reliable signal, but it is a city center location....
- Internet - 3/10 - 20 Euro for 24 hours, it worked for 3 hours, then blocked me. The helpline didn't help, and when it worked it was really slow.
- Conference facilities - 7/10. Good, high quality facilities, projectors, wireless networking in the meeting rooms, well equipped.
- Mushroom factor - 2/10 - A couple of polypores on the trees, but city centre (roll on Stromberg!)
-
RSC Chemical Tools Meeting, October 1st 2009, Stevenage, Herts
 Here is a link for a Royal Society of Chemistry (RSC) meeting held in Stevenage in October.
Here is a link for a Royal Society of Chemistry (RSC) meeting held in Stevenage in October.
The schedule is....
10.00 Herbert Waldmann MPI of Molecular Physiology, Dortmund, Germany
Bioactivity Guided Navigation of Chemical Space10.40 John Overington European Bioinformatics Institute, Hinxton, UK
ChEMBL - Open Access Databases and Tools for Drug Discovery11.20 Malcolm Young University of Newcastle, UK
Counterintuitive properties of complex networks in brains and cells12.00 Lunch
13.00 Chris Schofield University of Oxford, UK
The Hypoxic Response - from Molecules to Systems13:40 Albert Heck University of Utrecht, The Netherlands
Chemical Proteomics: Exploring the Role of Small Molecules in Molecular Systems Biology14.40 Seth Grant MRC Cambridge, UK
Synapse Systems Biology: New Models of Brain Organisation15.20 Glenn Prestwich University of Utah, Salt Lake City, USA
Engineered Extracellular Matrices for Drug Discovery and Reparative Medicine16.00 Round Table Discussion
17:00 Close
The picture above is of Stevenage town center, I have never seen the sky so blue there (I live nearby), maybe it is photoshopped?