-
2010 New Drug Approvals - part IX - Sipuleucel-T (Provenge)
On April 29th, the FDA approved Sipuleucel-T (Tradename: Provenge, REsearch Code: APC-8015) a highly novel treatment (a cell-based vaccine) for hormone refractory (castration resistant) prostate cancer. Patients with this form of late-stage prostate cancer have refractory metastases after hormonal therapy even though they may have few symptoms. Sipuleucel-T has had a complex development and approval history (as a google search will readily show). Sipuleucel-T is a mixture of white blood cells that are extracted from the patient through a process called Leukapheresis, which is routinely used to isolate white blood cells e.g. for diagnostic purposes. In the 3 days that pass between the extraction of the white blood cells from the patient and the treatment with sipuleucel-T, the mixture is activated by exposing the extracted cells to an engineered protein called PAP-GM-CSF. In this, PAP stands for prostatic acid phosphatase (Uniprot P15309), which is produced in high amounts by metastasized prostate carcinoma cells. Among the cells extracted from the patient are antigen presenting cells which internalize the protein and, after lysosomal processing, present fragments of the proteins in conjunction with the immune stimulatory MHC class II molecules. This complex has an activating effect on all immune cells that have been in contact with PAP. Thus, upon re-infusion, the autologous immune cells promote a clonal growth of immune cells that are specifically active against the PAP-antigen and coordinate an immune response against the tumor cells, which present PAP antigen in abundance. In clinical trials with 512 patients, sipuleucel-T treatment increased patient survival by 4.1 months on average compared to no treatment. Among reported averse side effect are chills, nausea, fatigue and pain. Sipuleucel-T is marketed by Dendreon under the name Provenge. The full prescribing information for sipuleucel-T can be found here. -
2010 New Drug Approvals - part VIII - Cabazitaxel (Jevtana)
ATC code (partial): L01CD
The FDA approved Cabazitaxel on June 17th. Cabitaxel is used in combination with prednisone to treat advanced, hormone-refractory prostate cancer.
Typically, prostate cancer occurs in older males and is among the most common forms of cancer affecting men with around 200,000 new cases per year diagnosed in the United States. Prostate cancer is a glandular cancer that is generally slow-growing. However, prostate cancer develops the ability to penetrate into other tissues and to form metastases.
Cabazitaxel is a new antineoplastic agent that inhibits the function of microtubules. Like other taxanes, it binds to beta-tubulin and promotes and maintains its incorporation in the assembled microtubule. As a consequence the dynamic structure of the microtubule cytoskeleton is 'frozen' and the concentration of free tubulin decreased. Mitotic cells, which depend on microtubules to restructure their shape and organelle organization, undergo apoptosis or stop progressing through the cell cycle. Thus, tumor growth is stalled.
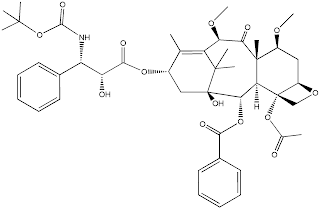
Chemically, cabazitaxel is a dimethylated variant of the state-of-the-art treatment docetaxel. Cabazitaxel is an example of a semin-synthetic natural product, with the key raw ingredient isolated from yew leaves. Cabazitaxel is a lipophillic molecule with a high molecular weight 835.93 g.mol-1).
The volume of distribution (Vss) is 4,864 L. In vitro, the binding of cabazitaxel to human serum proteins was 89 to 92%. Cabazitaxel is mainly bound to human serum albumin (82%) and lipoproteins (88% for HDL, 70% for LDL, and 56% for VLDL). Cabazitaxel is extensively metabolized in the liver (> 95%), mainly by the CYP3A4/5 isoenzyme, and to a lower extent by CYP2C8. Clearance (primarily as metabolites) is via both urine and feces. Cabazitaxel has a plasma clearance of 48.5 L.hr-1.
Clinical trials with showed a statistically significant increase in survival rate by 2.4 month.
Cabazitaxel is to be administered as an intravenous infusion of 25mg/mm2(body surface) every three weeks in combination with a daily regiment of 10mg prednisone.
Cabazitaxel comes with a black box warning (fatal neutropenia). Patients have to be monitored for changes in neutrophil counts and treatment appropriately adjusted if a drop below 1500 neutrophils/mm3 is observed. Other adverse side effects of cabazitaxel are hypersensivity reactions, gastrointestinal symptoms and renal failure.
Cabazitaxel is marketed by Sanofi-Aventis under the name Jevtana. This is a link to the full prescribing information.
SMILES:
CO[C@H]1C[C@H]2OC[C@@]2(OC(C)=O)[C@H]3[C@H](OC(=O)c4ccccc4)[C@]5(O)C
[C@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)c6ccccc6)C(=C([C@@H](OC)C
(=O)[C@]13C)C5(C)C)C
InChI:
1S/C45H57NO14/c1-24-28(57-39(51)33(48)32(26-17-13-11-14-18-26)46-40
(52)60-41(3,4)5)22-45(53)37(58-38(50)27-19-15-12-16-20-27)35-43(8,
36(49)34(55-10)31(24)42(45,6)7)29(54-9)21-30-44(35,23-56-30)59-25(2)
47/h11-20,28-30,32-35,37,48,53H,21-23H2,1-10H3,(H,46,52)/t28-,29-,30+,
32-,33+,34+,35-,37-,43+,44-,45+/m0/s1
InChI-Key:
BMQGVNUXMIRLCK-OAGWZNDDSA-N
Chemdraw: cabazitaxel.cdx -
Postdoc position - Target Discovery and Validation For Novel Malaria Drugs Using An Integrated Chemical Biology Approach
A number of you have asked for further details on the malaria postdoc project.... (details on how to apply, deadlines, etc can be found here).
Malaria remains one of the most significant causes of developing world mortality, and this global burden coupled with recent reports of parasites resistant to artemesinin, the most recently released anti-malarial, makes the search for new therapeutics an urgent priority. While the availability of multiple Plasmodium genome sequences has the potential to make a significant impact on malaria drug development, almost all previously successful therapies were empirically discovered and developed, and without a defined molecular target. Such entirely empirical-based discovery is now not considered practical, and so a hybrid approach of cell-based screening followed by target discovery for bioactive hit series is a compelling and practical way forward. Recently a number of very significant disclosures of HTS cell-based screening studies have occurred (see http://www.ebi.ac.uk/chemblntd). These data also typically have i) secondary assays to establish estimates of IC50 values, ii) cytotoxicity values against a human cell-line, and iii) a measure of compound ‘promiscuity’ (the propensity of a compound to be active in large number of assays, sometimes known as ‘frequent hitters’). Given that the compounds are cidal, and cell-penetrant, these datasets are highly valuable for future target and lead discovery for novel malaria therapies.
However, for these data, the precise molecular targets and mechanism of action are currently unknown, and a major challenge is in the identification of a target/mechanism of action for each compound/compound class. Once a specific target is known, it greatly simplifies future validation and compound optimisation, and also provides key insights into disease biology, pathogenesis, therapeutic index (TI), likelihood of resistance, and so forth.
The available postdoc position is multidisciplinary and highly collaborative and links computer-based target prediction and analysis along with experimental validation in order to identify potentially novel targets for the development of new and innovative anti-malarial therapies. The position will fund a postdoctoral position working jointly between two newly established groups, one (Overington, EMBL-EBI) with an expertise in chemoinformatics, drug discovery and lead optimisation, and the second (Rayner, Sanger) with expertise in malaria disease biology, genomics, experimental genetic manipulation and target discovery. Given the broad range of techniques required, we will provide training in any required areas.
The work will train a researcher in the principles of drug discovery as well as advanced molecular biology and genetic manipulation techniques in Plasmodium parasites, and will therefore give them a wide-ranging set of skills in the key future area of Chemical Biology.
The planned work programme is as follows:
- Clustering of compound series – each cluster will probably share a common mechanism of action.
- Selection of series for further detailed analysis.
- In silico prediction of molecular targets for selected compound clusters.
- Comparative genomic analysis of the predicted targets.
- in vitro validation of 40-50 predicted targets. This initial screen will be performed in the model organism P. berghei.
- In vitro validation of 5-10 high priority targets in P. falciparum parasites.
- For any validated targets, in vitro assays will be developed and structural collaborations explored to allow confirmation of binding/activity for selected targets.
- Clustering of compound series – each cluster will probably share a common mechanism of action.
-
June 2010 proposed INNs for protein kinase inhibitors
There is a new clutch of proposed protein kinase inhibitor INNs. These are:
Amuvatinib aka MP-470 and HPK-46, SuperGen's FLT3 inhibitor
S=C(NCc2ccc1OCOc1c2)N6CCN(c4ncnc5c3ccccc3oc45)CC6
Crizotinib aka PF-2,341,066, Pfizer's ALK and MET inhibitor
C[C@@H](Oc3cc(c2cnn(C1CCNCC1)c2)cnc3N)c4c(Cl)ccc(F)c4Cl
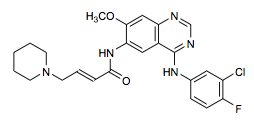
Dacomitinib aka PF-299,804 - Pfizer's EGFR, and HER2 inhibitor
COc3cc2ncnc(Nc1ccc(F)c(Cl)c1)c2cc3NC(=O)C=CCN4CCCCC4
Orantinib aka SU-6,668, Pfizer's Aurora, FGFR, VEGFR, and PDFGR inhibitor
Cc3[nH]c(C=c1c(=O)[nH]c2ccccc12)c(C)c3CCC(=O)O
Ruxolitinib aka INCB-18,424, Incyte's JAK2 inhibitor
C#CC[C@H](C1CCCC1)n4cc(c2ncnc3[nH]ccc23)cn4
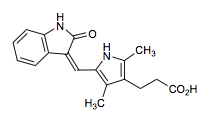
Silimitasertib aka CX-4,945, Cylene's CK2 inhibitor
O=C(O)c1ccc4c(c1)nc(Nc2cccc(Cl)c2)c3ccncc34
Tivantinib aka ARQ-197, Arqule's MET inhibitor
O=C4NC(=O)[C@@H](c1cn3CCCc2cccc1c23)[C@@H]4c5c[nH]c6ccccc56
-
Can't get enough of the world cup, and want to use the ChEMBL databases?
Here is a small slice of humor. I have been watching all the 2010 World Cup matches here in Korea, with Korean commentary (what day is it exactly?), but now when I turn the television off, the world sounds strangely quiet, unsettling, and to be perfectly frank, a little scary.
However, I've found a simple solution through the magic of teh interwebs (you will need your sound enabled on your computer).
-
Role of open chemical data in aiding drug discovery and design
We have just published a brief editorial in Open Chemical Data entitled 'Role of open chemical data in aiding drug discovery and design' in the journal Future Medicinal Chemistry, the good news is that the article is free, and downloadable here
%A A. Gaulton %A J.P. Overington %T Role of open chemical data in aiding drug discovery and design %J Fut. Med. Chem. %V 6 %P 903-907 %D 2010 %O ISSN 1756-8919
-
Postdoc position available - ChEMBL and malaria
There is an opportunity to apply for a Postdoc. position under the joint supervision of John P. Overington (EMBL-EBI) and Julian Rayner (Sanger Centre). The project will use the recently published and publicly released HTS Malaria datasets, and attempt to predict and validate target assignments using a variety of computational and experimental approaches. The position would ideally suit an ambitious and skilled researcher interested in chemical and molecular biology.
The deadline for applications is 15th August 2010. Further details and the application process can be found here and here.
I'd be happy to clarify anything about the project, just mail me.
-
ChEMBL Using Highcharts
 We have released and update to the ChEMBLdb Interface, the most significant of all the changes is the inclusion of the Highcharts javascript charting library. If you are not familiar with this library have a look at the demo page to get an idea of its capabilities. In the ChEMBLdb interface we used the library to update the Target Browser page and introduced a scatter plot view for compound result sets.
We have released and update to the ChEMBLdb Interface, the most significant of all the changes is the inclusion of the Highcharts javascript charting library. If you are not familiar with this library have a look at the demo page to get an idea of its capabilities. In the ChEMBLdb interface we used the library to update the Target Browser page and introduced a scatter plot view for compound result sets.
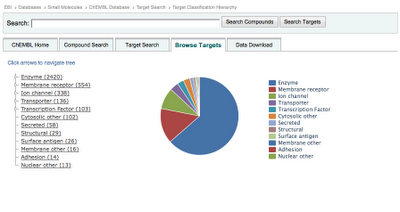
Target Browser Update
The new target browser can seen below:
Scatter Plot View
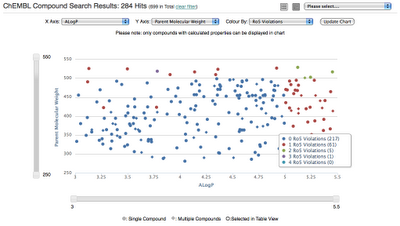
For compound searches returning less than 1000 compounds users can switch from a tabular view to a chart view:
 Chart features include:
Chart features include:
- Properties plotted on each axis can be updated, defaults to MW vs ALogP
- Compound colouring can updated, defaults number of Rule of Five violations
- Axis sliders can be used to 'zoom-in' to specific areas on the chart
- Clicking on a point in the scatter plot displays an image of the compound
We hope you like this addition to the interface and expect to see more Highcharts data visualisations soon.